A research team led by Professor Yusuke Matsuda, Assistant Professor Ginga Shimakawa (at the time of the study, currently at Kobe University), and Assistant Professor Yoshinori Tsuji (currently a junior associate professor) of the School of Biological and Environmental Sciences at Kwansei Gakuin University and Professor Genji Kurisu, Assistant Professor Akihiro Kawamoto, and Specially Appointed Associate Professor Christoph Gerle (currently at RIKEN) of the Institute for Protein Research at Osaka University, in collaboration with the University of Basel (Switzerland), Université Grenoble-Alpes (France), and other institutions, has announced that they discovered a novel protein allowing for highly efficient photosynthesis in marine diatoms. The research team elucidated the molecular mechanism underlying this highly efficient carbon dioxide fixation using genome editing and cryoelectron microscopy techniques. The results were published in Cell on October 2.
The secondary chloroplasts of diatoms are structurally distinct from those of land plants in that the CO2-fixing enzyme ribulose 1,5-bisphosphate carboxylase/oxygenase (Rubisco) assembles loosely in the chloroplast center to form structures called pyrenoids. Chloroplasts acquired via the symbiosis with blue-green algae (cyanobacteria) are called primary chloroplasts, and chloroplasts acquired via the symbiosis between a green or red alga with primary chloroplasts and another organism are called secondary chloroplasts. Diatoms have secondary chloroplasts of red algal origin.
Pyrenoids are traversed by thylakoid membranes in the center, and carbonic anhydrase (CA) is specifically found in the thylakoid lumen. CA is considered to rapidly generate CO2 from bicarbonate ions taken up by diatoms from seawater and stored in chloroplasts and supply CO2 to Rubisco, which aggregates in pyrenoids. Meanwhile, components and functions of pyrenoids were not well understood because they are difficult to isolate.
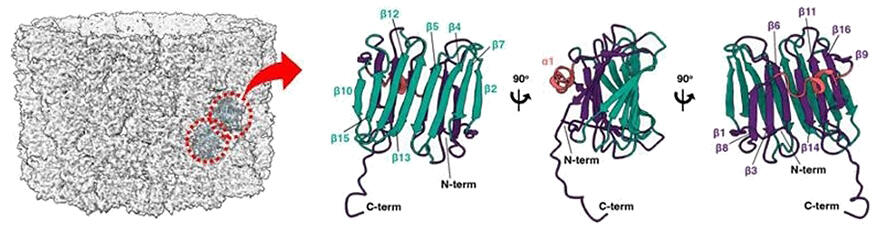
In this study, the research team used amino acids that can form covalent crosslinks between proteins interacting with each other in the cells upon UV irradiation. They searched for new proteins binding to Rubisco in diatoms that incorporated these amino acids into the protein synthesis system. As a result, a novel protein was discovered. The location of this protein within secondary chloroplasts was surveyed. It was found to be localized to the pyrenoid periphery and thus named PyShell (Pyrenoid Shell). The structure of PyShell prepared by genetic recombination was then analyzed using a state-of-the-art cryoelectron microscopy technique. PyShell self-polymerized in vitro, and cryoelectron microscopy analysis of the polymerized material revealed that it formed tube and sheet structures with periodicity.
The research team successfully determined the three-dimensional structure and found that the extended C-terminus connected PyShell monomers together to form tubes and sheets. Then, cryoelectron tomography was used to observe the microstructure in the diatoms in further detail. The same structure as observed with PyShell self-polymerized in vitro was observed around pyrenoids in chloroplasts. When the diatom PyShell genes were disrupted by genome editing, the gene-disrupted diatom mutants grew more slowly under atmospheric conditions, and its photosynthetic efficiency was markedly reduced to 1/80th of that of the wild strain.
When observing the secondary chloroplasts of PyShell gene-disrupted diatoms, normal pyrenoid structures could not be formed, the sheet structure surrounding the pyrenoid disappeared, and the pyrenoid structure was broken down and fragmented. The normal pyrenoid structure formed by PyShell was shown to be essential for CO2 supply to Rubisco. PyShell is conserved in diatoms and other algae that are secondary symbionts playing major role in marine primary production.
Provided by Kwansei Gakuin University
The study also confirmed that the PyShell gene is expressed in the oceans of the world by comparing sequences in a marine metatranscriptome database against the PyShell gene sequence. PyShell is considered to serve as a foundation of marine primary production.
Matsuda said, "I think PyShell is a very important factor to solve the mystery of chloroplasts playing an important role in the sea. The study started with the idea of isolating the pyrenoids without disturbing the structure somehow to learn more about the internal components, and it has taken a long time to get here. The advent of new technologies and national and international collaborations have been a great support. Meanwhile, this discovery is just the beginning. I consider this as a breakthrough to get to the essence of marine photosynthesis."
Journal Information
Publication: Cell
Title: Diatom pyrenoids are encased in a protein shell that enables efficient CO2 fixation
DOI: 10.1016/j.cell.2024.09.013
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




