A research group including Assistant Professor Jun Zhou, Researcher Zhengyu Zhao, and Professor Norio Shibata of the Department of Life Science and Applied Chemistry at Nagoya Institute of Technology, in collaboration with the University of Valencia (Spain), announced that they achieved a cross-coupling reaction of aliphatic fluorides and aromatic methane without using a transition metal catalyst. The strong carbon-fluorine (C-F) bond in PFAS can be efficiently cleaved to achieve a carbon-carbon cross-coupling reaction, and simultaneously cleaved fluorine moieties can be recovered as potassium fluoride. It is expected that this result will lead to environmentally friendly fluorine resource recycling. The results were published in the international journal Chemical Science on October 1.
Provided by Nagoya Institute of Technology
Transition metal-catalyzed cross-coupling reactions are commonly used in material syntheses. The use of these metals may lead to contamination by impurities. There is a need to develop a general-purpose method that does not use transition metals.
Organic fluorine compounds are used in a wide range of applications, from the medical field to the automotive industry and even for frying pan manufacturing, for example, as Teflon and other fluororesins. However, concerns about their persistence and accumulation in living organisms have led to a rapid movement in recent years to regulate them. Because C-F bonds are the strongest covalent bonds formed by carbon, their cleavage in the molecular transformation and coupling reactions of aliphatic fluorides has been considered difficult even with transition metal catalysts.
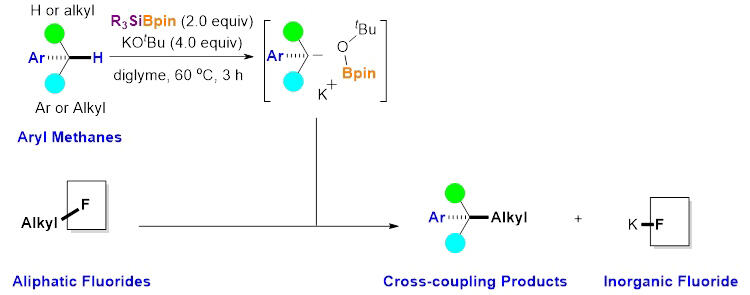
In this research, the group developed a new molecular conversion method that does not require transition metal catalysts and can synthesize aromatic methanes with highly complex structures in one step from aliphatic fluorides and aromatic methane. Aliphatic fluorides were used as reaction substrates instead of conventional aliphatic halides or their equivalents.
The researchers developed a unique reaction process that does not use any transition metals but instead combines boron, silicon, and potassium. The C-F bond, the strongest covalent bond formed by carbon, was successfully cleaved. Specifically, aliphatic fluorides and aromatic methane compounds were mixed in a diglyme solution and stirred with triethylsilyl-tetramethyl-dioxaborolan (Et3SiBpin) and potassium tert-butoxide at 60℃ for 3 hours. This simple operation can yield the cross-coupling products and potassium fluoride.
The method is independent of the structure of aliphatic fluorides and can be applied to commonly used aliphatic iodides, bromides, and chlorides. The cleavage of the C-F bond and the C-H bond of aromatic methanes is likely caused by ionic reactions rather than radical reactions. Because aliphatic fluorides and aromatic methanes are readily available due to the large number of commercial derivatives, more complex and diverse aromatic methane compounds are expected to be efficiently synthesized by freely combining the two substrates.
Shibata said, "Hydrocarbons containing fluorine are stable and robust. Thanks to this property, they support our daily lives; however, they are difficult to decompose in nature and tend to accumulate in the environment. We have previously discovered several techniques for breaking the carbon-fluorine bond, but the specific mechanisms by which this cleavage occurs remain unknown. This research allowed us to partially elucidate the decomposition mechanism. This achievement raises the possibility of applying the technology to the decomposition of PFAS, which has become a social problem, and to fluorine recycling technology."
Journal Information
Publication: Chemical Science
Title: A silylboronate-mediated strategy for cross-coupling of alkyl fluorides with aryl alkanes: mechanistic insights and scope expansion
DOI: 10.1039/D4SC04357J
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




