A research group including Professor Yoshinori Takashima of the Graduate School of Science and Professor Hiroshi Uyama of the Graduate School of Engineering at Osaka University, and Kyoeisha Chemical (President & CEO Kiyoo Kataoka) announced that they developed a biodegradable polymer by devising a new molecular design. The new polymer is eight times tougher than conventional polymers, while also allowing resource recycling using the biocatalyst lipase. By introducing a movable crosslink in which the polymer chain passes through the ring structure of cyclodextrin, they successfully improved the stability and extended the longevity of the material. The new polymer is expected to contribute to a resource-recycling society. The results are published in the October 29 issue of the international journal Chem.
Provided by Osaka University
Polymer materials, such as plastics, have become an issue in the realization of a recycling society, not only because their environmental proliferation has become a problem, but also because they emit a large amount of CO2 when incinerated. The realization of the three Rs is expected to be a solution to these issues: waste reduction (reduce) through toughing (improved stability and longevity), reformability (reuse), and reuse of used materials as resources (recycle).
Previously, Uyama and his colleagues reported that a mixture of lipase and poly ε-caprolactone (PCL) in an organic solvent can be used for enzyme-catalyzed degradation and repolymerization. Furthermore, the low molecular weight of the polymer material has posed challenges to its toughness, making it difficult to achieve degradability and toughness.
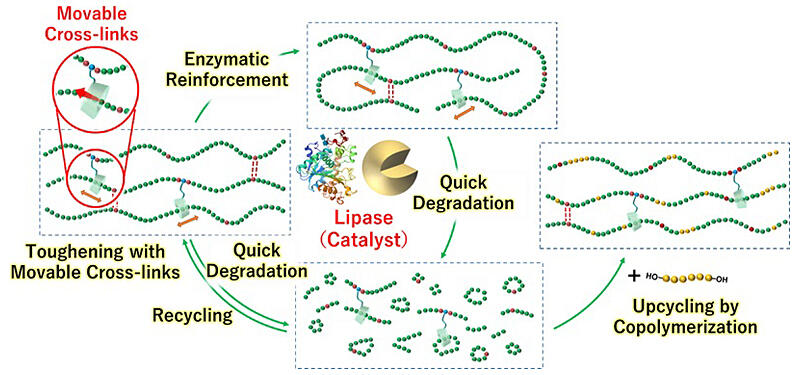
The research group employed this approach and devised a design that introduces a movable crosslink, which has been reported to improve toughness by dispersing stress. Specifically, a structure (CD diol) containing cyclodextrin (CD) with a ring structure was incorporated into the side chain of a degradable polymer (PCL-PU) comprising PCL and polyurethane (PU) by reacting it with isocyanate. PCL-PU passes through the ring structure of CD to provide mobility.
The prepared degradable polymer with movable crosslinks exhibited eight times greater toughness in mechanical evaluation tests than conventional PCL-PU. Repeated enzyme reactions and repolymerization increased the molecular weight and improved toughness. Moreover, degradation was accelerated by a factor of 20.
Because the degradation rate increased depending on the number of movable crosslinks introduced, interactions between the polymer chains were weakened, making it easier for lipase to react. Furthermore, it was confirmed that the low-molecular-weight decomposition products could be recycled via repolymerization. The researchers also confirmed the possibility of copolymerization with other types of polylactic acid and inorganic polymers, enabling the synthesis of polymers with different chemical properties.
Takashima said, "It is entirely possible to utilize this material in society as is, but there is a huge amount of polyester waste in society, especially from clothing. By mixing polyester with oligomers, which are the products of the decomposition of our material, we hope to create a new material with unprecedented chemical properties that is degradable and tough, yielding a long-lasting, degradable, and reusable polyester."
Journal Information
Publication: Chem
Title: Exploring enzymatic degradation, reinforcement, recycling, and upcycling of poly(ester)s-poly(urethane) with movable crosslinks
DOI: 10.1016/j.chempr.2024.09.026
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




