Water electrolysis is actively studied as an environment friendly hydrogen production technology. Iridium oxide is used as the electrode catalyst that promotes the oxygen evolution reaction at the anode. However, because iridium is a rare and expensive element, the development of alternative materials is essential for the widespread use of hydrogen. While there is technology that finds highly active catalysts, the supply of durable catalysts is limited. Therefore, if we can quantitatively predict the catalyst lifetime and efficiently search for highly durable materials, we can promote the development of alternative catalysts.
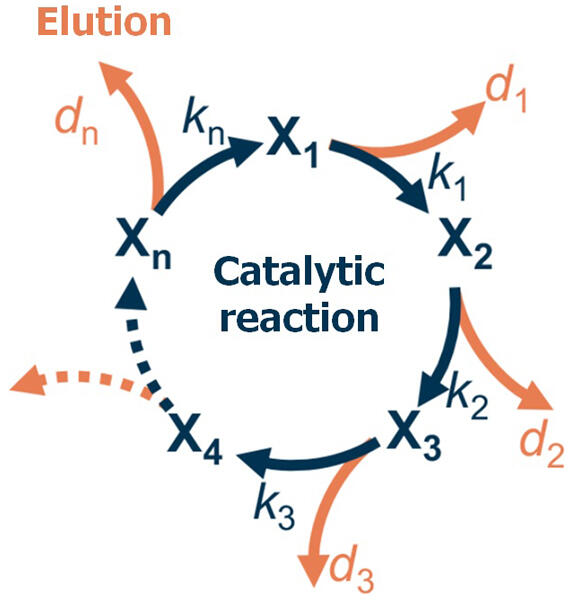
In their previous studies, a joint research group comprising Research Scientist Hideshi Ooka and Team Leader Ryuhei Nakamura of the RIKEN Center for Sustainable Resource Science revealed that a catalyst gradually dissolves during the reaction, causing its degradation. Furthermore, the researchers now succeeded in developing a mathematical model for predicting the catalyst lifetime from the elution rate.
Specifically, water was electrolyzed using a manganese oxide (MnO2) catalyst, and the elution rate and catalyst lifetime were measured under various reaction conditions to verify the validity of the developed equations. The derived theoretical lifetime was correlated to the lifetime measured in experiments, and the qualitative trends were theoretically reproduced, confirming the validity of the mathematical model. Furthermore, it was not possible to verify the quantitative agreement between the theoretical and experimental lifetimes because the rate constants could not be obtained in this experiment. In the future, improving the accuracy of mathematical models and theories that can predict catalyst lifetime will be essential.
Because a catalyst lifetime of several years is essential for the industrial use of electrocatalysts in hydrogen production, measurements require a long experimental time. However, if the time required to evaluate the catalyst lifetime can be reduced using mathematical models, highly durable materials can be developed in a short period of time. This is a step toward the industrial use of electrocatalysts in hydrogen production to achieve carbon neutrality.