Combining microRNAs in urinary exosomes can be used as a basis for early cancer detection. The method was developed by the research team led by Professor Takao Yasui of the School of Life Science and Technology at the Institute of Science Tokyo, in collaboration with Designated Professor Atsushi Natsume of the Institutes of Innovation for Future Society at Nagoya University; Professor Takeshi Yanagida of the Graduate School of Engineering at the University of Tokyo; Professor Kazuki Nagashima of the Research Institute for Electronic Science at Hokkaido University; Professor Takashi Washio of the Faculty of Business and Commerce at Kansai University; Yuki Ichikawa, CTO of Craif; Professor Takahiro Ochiya of the Institute of Medical Science at Tokyo Medical University; Specially Appointed Professor Tomoji Kawai of the Institute of Scientific and Industrial Research at Osaka University; and Professor Yoshinobu Baba of the Institute of Quantum Science at the National Institutes for Quantum and Radiological Science and Technology (QST). The method can distinguish cancer patients with a sensitivity of 98.2% and a specificity of 96.5% and identify early-stage cancer patients with an area under the receiver operating characteristic (AUROC) curve of 0.987. The work was published online in Analytical Chemistry.
Provided by Science Tokyo
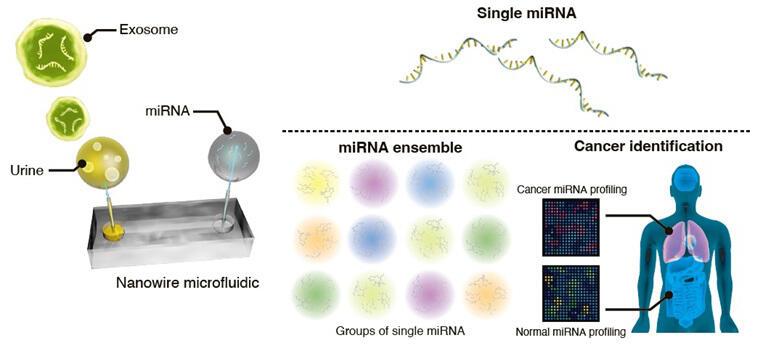
Exosomes, which are microparticles measuring about 40−200 nanometers in diameter, are released by all cells and serve as information carriers in cell-to-cell communication in the body. MicroRNAs, which are pieces of information carried by exosomes released by cancer cells, are considered to be signs associated with cancer in the body.
The research group constructed urinary microRNA ensembles (combinations of microRNAs) through analysis of urinary microRNAs and machine learning-based analysis, aiming for noninvasive early detection of cancer. MicroRNAs circulating in the blood are mostly encapsulated in exosomes, which are well-known information carriers in body fluids. In view of the length of time required for exosome movement in the blood and the length of time required for blood circulation, not all exosomes in the blood are used for cell-to-cell communication during single blood circulation. This suggests that unused exosomes are nonselectively filtered out by the kidneys.
Based on this hypothesis, the research group considered the possibility that urine may contain exosomes derived from cancer cells. Increasing the number of microRNA species detected is important for constructing a urinary microRNA ensemble. While more than 2,000 microRNA species in blood have been reported, the paucity of reported urinary microRNA species hampered ensemble construction. Based on the idea that the paucity of microRNA species detected in urine is attributable to the inability to capture urinary microRNA species comprehensively, the research group made full use of nanowires and discovered that the number of microRNA species in urine was comparable to that in blood.
Previously, the group succeeded in comprehensively capturing exosomes through the Coulomb force and hydrogen bonding using single-crystal zinc oxide nanowires and in discovering more than 1,300 microRNA species in a milliliter of urine through microRNA analysis of urinary exosomes. In this study, they prepared zinc oxide nanowires and analyzed 200 urine samples (100 from noncancer subjects and 100 from cancer patients) to confirm the number of human microRNA species in urine.
The results revealed that many urinary microRNA species were associated with a person's lifestyle habits (such as smoking and alcohol consumption), with a breakdown of 1,300 microRNA species varying from human to human. Since the number of urinary microRNA species was found to be comparable to that of blood microRNA species, they used the data of urinary microRNAs extracted and analyzed from cancer and noncancer patients for machine learning-based analysis of urinary microRNA data from 200 people to construct a urinary microRNA ensemble capable of distinguishing cancer patients from noncancer patients. They found that the constructed ensemble could be used to characterize the urinary microRNA patterns of cancer and noncancer patients whose samples were not used in the ensemble construction in terms of whether they are closer to cancer or noncancer patients' urinary microRNA patterns (sensitivity 98.2% and specificity 96.5%). Furthermore, they constructed another urinary microRNA ensemble using machine learning-based analysis of urinary microRNAs from cancer patients and demonstrated that it could be used to identify early-stage cancer patients (stage I) with an AUROC curve of 0.987.
In addition to discriminating lung cancer, brain tumors, and noncancer, this study also showed the possibility of discriminating 11 groups composed of lung cancer, colorectal cancer, endometrial cancer, renal cancer, leukemia, lymphoma, pancreatic cancer, prostate cancer, gastric cancer, urothelial cancer, and noncancer patients. The research group hopes to advance toward the development of technology to ensure early cancer detection through the comparison of microRNA ensembles before and after the onset of cancer and the expansion of detectable cancer types.
Journal Information
Publication: Analytical Chemistry
Title: Early Cancer Detection via Multi-microRNA Profiling of Urinary Exosomes Captured by Nanowires
DOI: 10.1021/acs.analchem.4c02488
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




