A research team including Professor Kazuhiko Maeda and Researcher Chomponoot Suppaso of the Department of Chemistry, School of Science at the Institute of Science Tokyo, established a new synthesis method for preparing coordination polymers containing lead-sulfur bonds. Further, using this polymer as a solid photocatalyst, they succeeded in increasing the efficiency of converting CO2 into formic acid under visible light by approximately 10 times compared to conventional methods. The results were published in Advanced Functional Materials.
Provided by Science Tokyo
If CO2 can be converted into useful substances via the use of light and electricity derived from renewable energy sources, this will be a major step toward the realization of a carbon circulation society. In a previous study, the research team discovered that coordination polymers containing lead-sulfur bonds activated by visible light can act as a photocatalyst that converts CO2 into formic acid using light energy. However, the utilization efficiency of electrons and holes produced in such coordination polymers using visible light is low, and the apparent quantum yield is <3%.
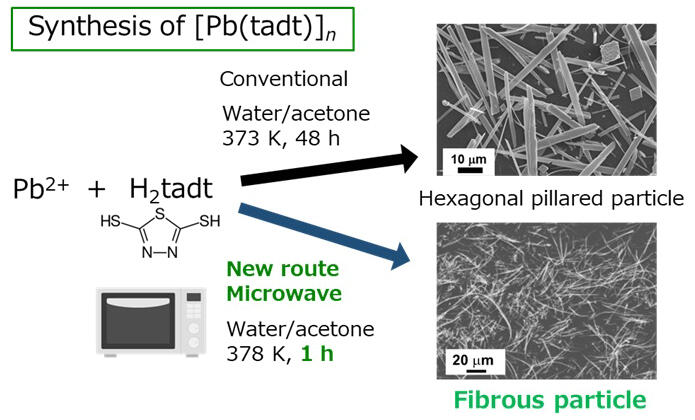
In this study, the researchers found that fibrous coordination polymers can be synthesized via a microwave-assisted solution method. On optimizing the synthesis temperature and time, this fibrous coordination polymer exhibits a higher specific surface area and fewer surface defects that are the centers of electron and hole recombination than columnar coordination polymers synthesized via conventional methods. The prepared fibrous coordination polymer can convert CO2 to formic acid with a high apparent quantum yield of 25% (irradiation wavelength: 400 nm) while maintaining a high formic acid production selectivity of over 99%. This value is the world's highest among photocatalysts that convert CO2 to formic acid under visible light.
Furthermore, when this coordination polymer was fixed on a conductive substrate and used for CO2 electrolysis, CO2 is converted to formic acid at a high current density of 300 mA per square centimeter. The faradaic efficiency of formic acid production in this case exceeds 90%, demonstrating that the coordination polymer can maintain high formic acid production selectivity even under fast electrolysis conditions.
In addition, with conventional lead-based electrode catalysts, hydrogen production through water reduction occurs simultaneously when high current is applied, reducing the selectivity of CO2 reduction. However, when the coordination polymer was used, hydrogen production hardly occurred, making it useful as an electrode catalyst for CO2 reduction.
In the future, other materials will be explored, and synthesis methods will be optimized for constructing new catalytic systems that can be driven by small amounts of energy. Selective formic acid production at a current density of 300 mA per square centimeter, which is required for practical formic acid production through CO2 electrolysis, was realized, opening a new direction for CO2 reduction using electrical energy.
Journal Information
Publication: Advanced Functional Materials
Title: Fibrous Pb(II)-Based Coordination Polymer Operable as a Photocatalyst and Electrocatalyst for High-Rate, Selective CO2-to-Formate Conversion
DOI: 10.1002/adfm.202417223
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




