A research group led by Professor Tohru Ishitani and Specially Appointed Assistant Professor Kana Aoki of the Research Institute for Microbial Diseases at Osaka University, in collaboration with the Medical Institute of Bioregulation at Kyushu University, has announced their research results showing that tension in the cell population constituting the animal body plays a function in detection and elimination of unfit cells. Using zebrafish, they found that neighboring fit cells sense unfit cells via changes in intercellular tensile force and induce cell apoptosis. They also confirmed that cells were misaligned and formed tumors when this function was defective. The findings are expected to contribute to elucidating the pathophysiology of and understanding the defense mechanisms against cancer and other diseases caused by defects of this function. The results were published in the November 15 issue of the international journal Science Advances.
Provided by the Department of Immunoparasitology, Research Institute for Microbial Diseases, Osaka University
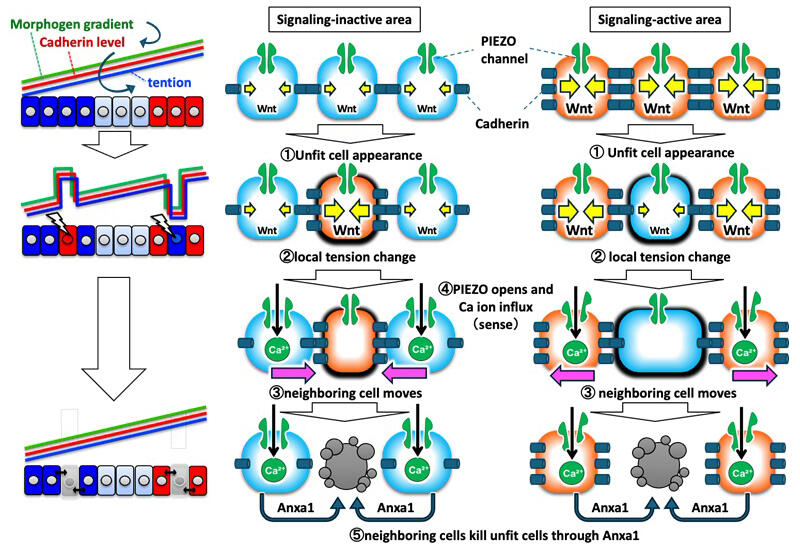
To maintain a healthy body, an appropriate number of cells with specific functions must be put in the proper locations. Such cellular arrangement is created by a chemical signaling activity gradient called the morphogen gradient. For example, the cellular arrangement along the anterior-posterior axis of vertebrate embryos is formed based on the Wnt morphogen gradient, a type of morphogen gradient. The Wnt proteins are secreted from the posterior region of early embryos, and the Wnt signaling (chemical signaling) is more strongly activated in the posterior region closer to the site of secretion. The result is the formation of a morphogen gradient, along which each cell keeps track of its location, and gene expression in each cell is induced. It has also been reported that cells disrupting the Wnt morphogen gradient undergo malignant transformation.
In 2019, Ishitani and his colleagues developed a technique for real-time visualization of the Wnt morphogen gradient formation process in zebrafish. They have shown that unfit cells incapable of properly activating Wnt signaling occur frequently, even in normal embryos, and are eliminated through cell apoptosis. They have also shown that the strength of Wnt signaling is converted to the amount of the cell adhesion molecules cadherins in the plasma membrane and that cadherins are essential for eliminating unfit cells. Meanwhile, it was unclear how unfit cells were sensed and removed.
Cadherins are transmembrane proteins, and their extracellular domains mediate cell adhesion by binding to cadherins of neighboring cells, while their intracellular domains generate tensile force by binding to the actomyosin complex. Therefore, the research group investigated the possibility that changes in tension are utilized to detect abnormal cells based on observation of the beating of abnormal cells. They tested the hypothesis that the Wnt signal strength is converted to the cadherin level in the plasma membrane and finally to the tensile force in zebrafish embryos. They confirmed that the morphogen gradients created tension gradients through multiple experiments, including tension measurement after laser cutting at positions differing in the Wnt signal.
It was found that calcium ions flowed exclusively into cells adjacent to cells with inappropriate Wnt signaling for their locations (unfit cells) through a calcium ion channel (Piezo1) that is opened and closed by physical stress on the plasma membrane. They also confirmed that a Piezo family channel inhibitor blocked the calcium ion influx and eliminated unfit cells. Furthermore, they analyzed gene expression changes caused by the calcium ion influx in cells adjacent to unfit cells.
Annexin A1 was found to be expressed specifically in adjacent cells. They confirmed that inhibition of annexin A1 also prevents unfit cells from undergoing cell apoptosis. Annexins cause cell apoptosis by degrading cell apoptosis inhibitor proteins. Moving forward, the group will verify whether a similar mechanism plays a function in humans.
Journal Information
Publication: Science Advances
Title: Mechano-gradients drive morphogen-noise correction to ensure robust patterning
DOI: 10.1126/sciadv.adp2357
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




