A research group led by Associate Professor Masakazu Matsumoto of the Research Institute for Interdisciplinary Science at Okayama University and Professor Kenji Mochizuki of Zhejiang University in China announced that simulations of the freezing process of nanoscale water droplets have revealed that pentagonal bipyramidal− or icosahedral-shaped ice grains are produced at a certain rate. This result may be useful for the molecular design of "nucleating agents," which are sprayed on clouds to promote the formation of ice nuclei for artificial rainfall. The results are published in the October 18 issue of ACS Nano, a journal of the American Chemical Society.
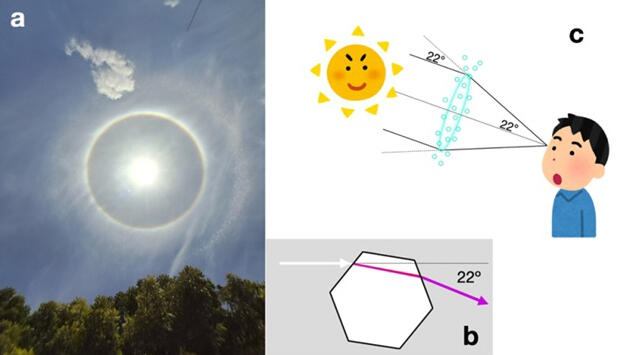
(b) Hexagonal columns of ice refract light and bend it 22°.
(c) Light bent by ice crystals reaches the observer from a direction 22° away from the sun.
Photo by Digi Venkat CC BY SA 4.0
Hexagonal snow crystals are formed by the growth of small ice grains in clouds as they fall, and snowflakes have a hexagonal shape because water molecules in crystals are arranged in a hexagonal pattern.
The shape of newly formed ice grains can be estimated from the ground by observing the distance of the sun's halo, an atmospheric optical phenomenon, from the sun's direction. The halo is caused by ice crystals in clouds refracting sunlight at high altitudes. In most cases, the halo is observed as a round ring with the sun at its center at an angle of 22° or 46°. From this angle, it is inferred that the angle between the faces of ice grains is 60° or 90°, indicating that ice grains exhibit a hexagonal shape (hexagonal pillars).
Furthermore, it has been reported that on rare occasions, a 28° halo (Scheiner's halo) can occur, and the angle between the faces of ice grains is approximately 70°. Twin crystals, in which two snowflakes are attached to each other at 70°, are often found in snow crystals falling on the ground and in artificial snow made in laboratories. This result indicates that two snowflakes do not stick together later but rather that two crystals, which are inclined at only 70° to each other from the time they form, grow simultaneously, suggesting that the initial ice grains have a 70° plane. Regular octahedral crystal grains of cubic ice have 70° planes, which has been thought to be the origin of the 70° planes observed in twin crystals and Scheiner's halo. However, the details have remained unclear.
Therefore, in this study, the freezing process of nanoscale water droplets was investigated in detail through computer simulation. The results revealed that pentagonal bipyramidal and icosahedral ice grains with 70° angles form at a certain rate. Both these shapes are formed by the merging of multiple tetrahedral cubic ice crystals. Pentagonal bipyramidal and icosahedral shapes appear as regular pentagons when viewed from certain angles.
Although ice crystals are very small, the ice surface structure is a factor that determines crystal stability. Pentagonal bipyramidal and icosahedral ice crystals are slightly more internally unstable than hexagonal crystals but can expose more stable surface structures, and such ice crystals are expected to form at a certain percentage. Ice microcrystals with unexpected shapes found in clouds can be observed in Antarctic ice, water soaked in materials with nano-sized cavities, comets, glaciers, and white smoke produced when dry ice is thrown into water.
Mochizuki said, "Ice we see in everyday life is technically called ice Ih. It has long been thought that ice Ic, which is slightly different from ice Ih, may exist in clouds. However, we could not observe it directly. Using molecular simulations, we predicted the pentagonal bipyramidal and icosahedral shapes of ice Ic collections and showed that these shapes explain the shape of polycrystalline snow. We will continue to unlock the wonders of water and ice, which are most familiar to humankind."
Journal Information
Publication: ACS Nano
Title: Multitwinned Ice Nanocrystals
DOI: 10.1021/acsnano.4c07226
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




