A research group led by Senior Staff Scientist Junji Koya (also a senior assistant professor at Keio University), and Chief Keisuke Kataoka (also a professor at Keio University), of the Division of Molecular Oncology at the National Cancer Center Research Institute, has announced that they developed a novel mouse model of natural killer (NK) cell lymphoma and clarified the pathogenic mechanism. The mouse model showed pathological features similar to extranodal NK/T-cell lymphoma, nasal type (ENKTCL), and this analysis revealed that the cellular origin of human NK cell lymphoma is tissue-resident NK cells. They also confirmed that treatments targeting proteins expressed specifically in tumors and other molecules prolonged survival. These findings are expected to contribute to developing novel therapeutic strategies for NK cell lymphoma and were published in the international journal Nature Communications on October 22.
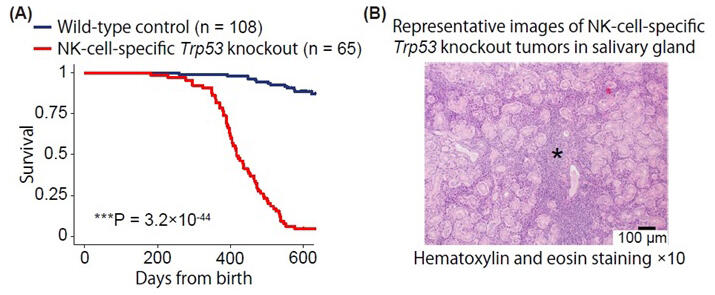
(A) Survival curves of NK cell-specific Trp53-deficient mice are shown.
(B) Histopathological image of salivary gland. Large, atypical tumor cells (marked with *) show marked infiltration within the salivary gland tissue. These features are similar to human ENKTCL.
Provided by the National Cancer Center
Lymphoma is a disease in which lymphocytes undergo malignant transformation and are histologically classified into Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL). ENKTCL occurs in extranodal organs and is caused by persistent infection with the Epstein-Barr (EB), a herpesvirus, from childhood to adulthood. It is a rare disease found particularly frequently among Asians, including the Japanese, and people in South America, and characterized by malignant tumors developing mainly in the nasal cavity and pharynx. ENKTCL poorly responds to anticancer drugs due to overexpression of P-glycoprotein, which is involved in drug resistance, and has a poor prognosis. Thus, the development of new therapeutic agents is needed. Abnormalities of TP53 and various other tumor suppressor genes have been reported in this disease, with the TP53 gene being most frequently affected. Meanwhile, the roles of TP53 abnormalities and EB virus in the pathogenesis remained unknown.
In this study, the research group developed a mouse model that develops NK cell lymphoma in the hematopoietic system and salivary glands, by deleting the tumor suppressor gene Trp53 in an NK cell-specific manner. In this mouse model, all individuals developed lethal NK cell lymphoma, with the onset being after surviving for over 1 year in more than 80% of the mice. Moreover, tumors were found to occur frequently in the salivary glands in addition to the spleen and lymph nodes, showing pathological features similar to human ENKTCL. These mice also showed a specific increase in NK cells with somewhat immature characteristics, similar to NK cells that later became tumors. This result indicates that tissue-resident NK cells, which settle and work in specific tissues and play an important role in tissue immune function, may be the origin of NK cell lymphomas.
The research group found that tumor development was noticeably accelerated in model mice expressing the LMP1 protein, which is produced by the EB virus. The model reproduced human ENKTCL characteristics, such as tissue necrosis and abnormal proliferation of blood vessels, and was confirmed to more closely resemble the actual pathology. The LMP1 expression by EB virus was also shown to alter the tumor immune microenvironment and accelerate tumor development. Furthermore, they found two important molecules of which therapeutic targets are lymphoma cells themselves. The first one is the Myc gene, which is known as an oncogene. The copy number of the Myc gene was increased in model mouse tumors, particularly in the LMP1-expressing model mice. The second one is a molecule called KLRG1. Detailed expression analysis of the KLRG1 gene in normal and malignant NK cells revealed that this molecule was expressed specifically in the malignant cells. For both molecules, they confirmed that the patients showed the same characteristics. Based on these findings, a new treatment combining an inhibitor that suppresses the MYC function and a therapeutic agent targeting KLRG1 was tested in mouse experiments and was shown to inhibit tumor growth.
Koya said, "There were many difficulties in developing this mouse model, such as the need for long-term observation for more than a year before the tumor development and the need to identify abnormalities in the salivary glands, which are difficult to detect with routine tests. However, the results of this study have clarified the pathogenic mechanism of NK cell lymphoma and demonstrated its potential as a new therapeutic target. In particular, the combination of therapies focusing on both cancer cells and the surrounding immune environment is promising. We hope that clinical trials of this approach will lead to improved treatment outcomes for this difficult disease."
Journal Information
Publication: Nature Communications
Title: Modeling NK-cell lymphoma in mice reveals its cell-of-origin and microenvironmental changes and identifies therapeutic targets
DOI: 10.1038/s41467-024-53376-1
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




