A joint research group led by Associate Professor Satoru Okuda of the Nano Life Science Institute (WPI-NanoLSI) (concurrently at the Sapiens Life Sciences, Evolution and Medicine Research Center), Kanazawa University, and Graduate Student Aki Teranishi of the Division of Nano Life Science, Graduate School of Frontier Science Initiative, Kanazawa University, has announced that they elucidated how epithelial folds are formed irreversibly by an L-shaped bracket-like integrated structure of actin molecule (one of the molecules that make up the cytoskeleton) during development. They found that epithelial fold irreversibility is achieved by the formation of the L-shaped actin bracket structure following the detection of the duration and amount of epithelial tissue deformations by cells. The findings are expected to contribute to the fields of tissue engineering and regenerative medicine. The results were published in the international journal Nature Communications on December 12.
© 2024 Satoru Okuda, Kanazawa University
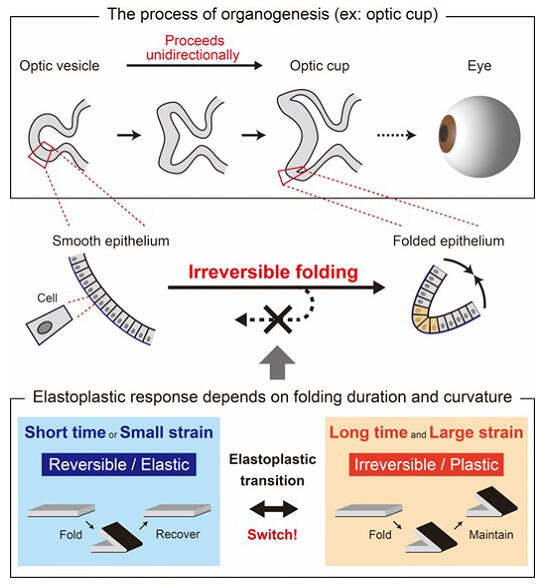
During the developmental process of organ formation, sheets of epithelial tissue (epithelial sheets) are intricately folded like origami to form the "shapes" of various organs. Once formed on the epithelial sheet during this process, the epithelial folds are known to be irreversible and never return to the original sheet structure. Many organs, such as the brain and eye, have numerous folds, most of which are formed during development and maintained after the body's growth. The irreversibility of these folds is an essential property for the proper formation of the organs. The formation of folds is deeply involved in the development of organs into specific shapes. Much work has been done on how folds formed in simple epithelial sheets are maintained.
Previous studies have shown that actin and myosin molecules (motor proteins), which make up the cytoskeleton, come together inside the folds of the epithelial sheet to generate contractile forces, which bend the epithelial sheet in one direction. Meanwhile, the mechanism of folding irreversibility remained unknown.
In this study, the research group independently developed a technology to apply complex three-dimensional deformation to living epithelial sheets by combining cell culture and micromanipulation techniques. This technology has made it possible to measure the elastoplasticity of epithelial sheet folding. Experimental results revealed that epithelial sheet folding became irreversible in a switch-like manner depending on the "duration" of deformation application and "amount of deformation." They also found that the folds returned to their original shapes when the duration or amount of deformation was less than a threshold. Furthermore, two molecular signals were activated when the amount of deformation applied to the epithelial sheet exceeded a threshold.
These two molecular signals are essential for elastoplastic (property of having both elasticity and plasticity; the former is a property of being deformed when a force is applied but returning to its original shape when the force is removed, and the latter is a property of being deformed when a force is applied and not returning to its original shape when the force is removed) transition. The results of the study revealed that cells sense the deformation applied to the epithelial sheet through these signals. Cells were found to regulate folding irreversibility by sensing the duration and amount of deformation applied to the epithelial sheet via molecular signals and forming actin brackets.
Okuda said, "In origami, folding paper naturally forms folds, but we rarely pay attention to them. Similarly, the folds also occur naturally in biological tissue, and our discovery has revealed that the folds are skillfully controlled by the cells. This fact makes us feel the precise and flexible mechanisms behind life."
Journal Information
Publication: Nature Communications
Title: An actin bracket-induced elastoplastic transition determines epithelial folding irreversibility
DOI: 10.1038/s41467-024-54906-7
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




