A research group led by Research Associate Sharmin Naher, Senior Lecturer Takako Kikkawa, and Professor Noriko Osumi at Tohoku University Graduate School of Medicine, in collaboration with the Institute of Development, Aging, and Cancer and the Frontier Research Institute for Interdisciplinary Sciences of Tohoku University, the National Institute of Neuroscience of National Center of Neurology and Psychiatry, and the National Yang Ming Chiao Tung University in Taiwan, has announced that they have identified Kif23 protein, which acts as a motor molecule, as a novel molecule causing microcephaly. This discovery was made using embryonic mouse brains to study the effects of a KIF23 gene mutation found in microcephaly patients. The researchers confirmed that inhibition of the function of this molecule caused abnormal division of neural stem cells, resulting in cell death. The findings are expected to provide a foundation for future gene therapy against microcephaly and were published in the international journal The EMBO Journal on December 4.
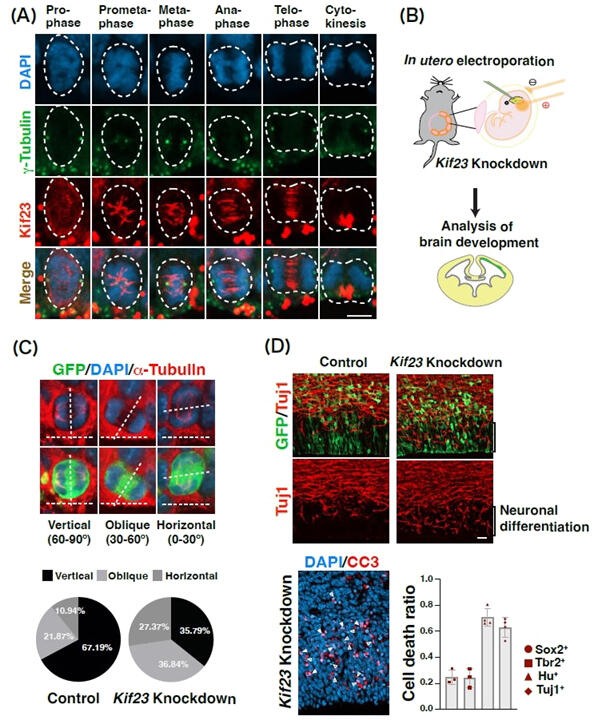
(A) Localization of Kif23 in neural stem cells. The cell division process consists of several stages: prophase, prometaphase, metaphase, anaphase, and telophase. The spindle is formed during prometaphase, and Kif23 begins to localize to the spindle. Kif23 (red), γ-tubulin (green): marker for centrosomes (which move to both poles of the cell during division), DAPI (blue): nucleus
(B) In vivo knockdown of Kif23 in the embryonic mouse brain using in utero electroporation.
(C) When Kif23 is knocked down, the division plane of neural stem cells is tilted more horizontally compared to the control group. α-tubulin (red): marker for microtubules that form the spindle
(D) Knockdown of Kif23 abnormally enhances neuronal differentiation and increases cell death of differentiated neurons. Tuj1 (red): neuronal marker, CC3 (red): cell death marker, Sox2 (circle): neural stem cell marker, Tbr2 (square): intermediate proliferative cell marker, Hu (triangle): neuronal marker, Tuj1 (diamond): neuronal marker
Provided by Tohoku University
Microcephaly is a condition in which the head of a newborn is noticeably smaller than the average size or stops growing after birth. It occurs in one in several thousand newborns and causes patients various disorders, such as epilepsy, cerebral palsy, and learning disabilities. It is caused by genetic abnormalities, intrauterine infections, exposure to harmful chemicals, and severe malnutrition during fetal life, and currently no cure has been found.
Neural stem cells proliferate and differentiate into neurons, which no longer proliferate. Brain development begins in the fetal period, in which neural stem cells divide, proliferate, and at the right time, differentiate into neurons, and results in a size increase. Because differentiation into neurons is completed in early fetal life, microcephaly is known to be caused in the very early fetal period, but details remain largely unknown.
Previous studies have reported that many of the mutated genes found in microcephaly patients are genes involved in cell division. Meanwhile, mutations in the KIF23 gene have been reported in patients with microcephaly, but details have remained unknown. The protein encoded by this gene has been shown to be one of the motor molecules (kinesin proteins) that transport intracellular substances on a microtubule scaffold.
In the present study, the research group used embryonic mouse brains to investigate where and when the kif23 protein is distributed in neural stem cells during cell division. The results showed that the Kif23 protein was localized to the spindle. To investigate the effects of this protein on the development of the embryonic mouse brain in vivo, they used in-utero electroporation and RNA interference to inhibit the function of the protein.
The results showed that differentiation into neurons was enhanced by 20%-30% compared to the normal level, indicating a transient increase in the number of neurons. Furthermore, in mouse embryos treated to inhibit the Kif23 protein's function, cell death occurred only in neurons. Cell death was considered to have occurred due to abnormal cell division. Then, they introduced a human KIF23 mutant gene into mice treated to inhibit the Kif23 function and forcibly expressed the mutant Kif23 protein to determine whether the KIF23 gene mutation occurring in human microcephaly patients causes microcephaly.
As a result, they confirmed that there was an increase in differentiation into neurons and found that the increase was suppressed by the introduction of the normal human KIF23 gene. However, the increase in differentiation into neurons was not sufficiently suppressed when the human microcephaly-type mutant KIF23 gene was introduced.
Kikkawa said, "Although no cure for microcephaly has been established, our findings are expected to serve as a target for the treatment of microcephaly in the future when gene therapy is more developed."
Journal Information
Publication: The EMBO Journal
Title: Kinesin-like motor protein KIF23 maintains neural stem and progenitor cell pools in the developing cortex
DOI: 10.1038/s44318-024-00327-7
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




