A research group led by Specific Associate Professor Kentaro Yamamoto (currently associate professor at the Faculty Division of Engineering, Nara Women's University), and Professor Yoshiharu Uchimoto, of the Graduate School of Human and Environmental Studies at Kyoto University, in collaboration with Toyota Motor Corporation, the University of Tokyo, the University of Hyogo, Tohoku University, and the Institute of Science Tokyo, has successfully developed a new high-capacity intercalation cathode material. The developed material can be used in all-solid-state fluoride-ion batteries, which are expected to become next-generation secondary batteries beyond lithium-ion batteries. Their capacity values are more than twice those of lithium-ion battery cathodes. This is the first report in the world on a super-ceramic cathode material utilizing the valence change in nitrogen, which is a negative ion. Its ability to exhibit much higher capacity than conventional materials indicates the possibility of a revolution in storage batteries. This study is published online in the Journal of the American Chemical Society.
previously reported.
Provided by Nara Women's University
One way to develop a secondary battery with energy density surpassing current lithium-ion batteries is to perform multi-electron reactions at the positive and negative electrodes. However, multicharged ions, such as magnesium and aluminum ions, exhibit many kinetic disadvantages, including slow diffusion within a solid. When fluoride ions, which are monovalent anions, are used as carriers, they have a small radius, almost the same as the ionic radius of oxide ions. However, they allow fast ionic conduction in solids because they are monovalent. Safety can also be improved by employing nonflammable solids as electrolytes. Hence, all-solid-state fluoride-ion secondary batteries that use fluoride ions as carriers are attracting attention.
Metal/metal fluorides have been conventionally developed as cathode materials for all-solid-state fluoride-ion secondary batteries. However, these cathode materials demonstrate poor cycle and input/output characteristics due to the large volume change occurring during charging/discharging, i.e., fluorination/defluorination. Therefore, cathode materials that utilize topotactic fluoride-ion intercalation reactions, similar to electrode materials used in lithium-ion secondary batteries, are being developed to solve problems associated with the cycle and input/output characteristics of electrode materials. While these materials dramatically improve the cycle and input/output characteristics compared to metal/metal fluorides, they face the problem of smaller available capacity. To solve this problem, the group led by Yamamoto and Uchimoto has been developing acid fluoride cathodes that use oxygen molecular bond formation. However, they have not been able to obtain capacities that exceed those of cathode materials used in lithium-ion secondary batteries.
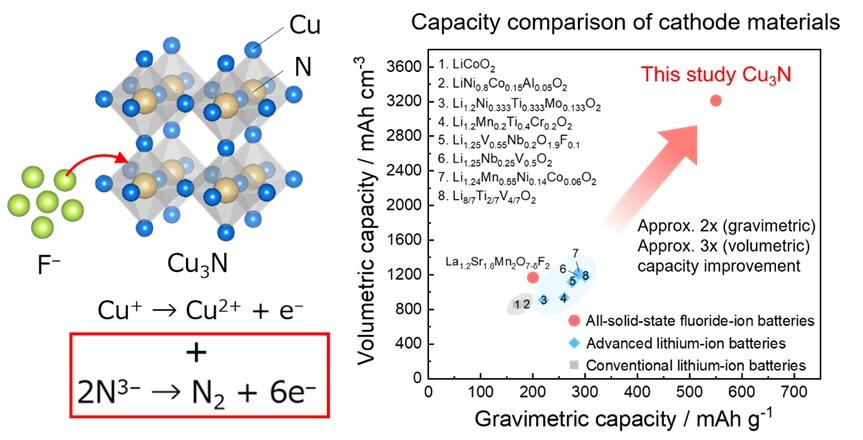
The research group has found that Cu3N nitride with an inverse ReO3−structure can reversibly insert much more fluoride ions than expected from its crystal structure and exhibits a high reversible capacity of 550 mAh/g, which is well over double that of existing lithium-ion secondary battery cathode materials. Moreover, using various analytical techniques, such as X-ray absorption spectroscopy and resonant inelastic X-ray scattering, at SPring-8, the reaction mechanism of the Cu3N cathode was analyzed from various perspectives. The analysis revealed that nitrogen, which is a negative ion, in addition to transition metal, which is a positive ion, were responsible for charge compensation when fluoride ions were inserted. It was also revealed that nitrogen forms molecular nitrogen in the cathode structure during charge compensation, facilitating the insertion of a much higher number of fluoride ions than expected from the crystal structure. This reaction mechanism is responsible for the high capacity of the Cu3N cathode.
In the future, intercalation cathode materials with even higher capacity than Cu3N and all-solid-state fluoride-ion secondary batteries with high energy density fabricated using these materials are expected to be developed by controlling the insertion and desorption reactions of a large number of fluoride ions by taking advantage of molecular nitrogen formation in the cathode structure.
Journal Information
Publication: Journal of the American Chemical Society
Title: Cathode Design Based on Nitrogen Redox and Linear Coordination of Cu Center for All-Solid-State Fluoride-Ion Batteries
DOI: 10.1021/jacs.4c12391
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




