A research group led by Designated Assistant Professor Sayuri Higashi at Gifu University's Institute for Advanced Study and Professor Seraphine V. Wegner at the University of Münster (Germany) has announced that they established a technique to control the activity of metalloenzymes in giant liposomes and succeeded in developing artificial cells that follow different fates depending on external metal ion stimuli. This technique may be useful for designing "pluripotent artificial cells" that mimic cell differentiation. The results were published in the international journal Nature Chemistry on December 23.
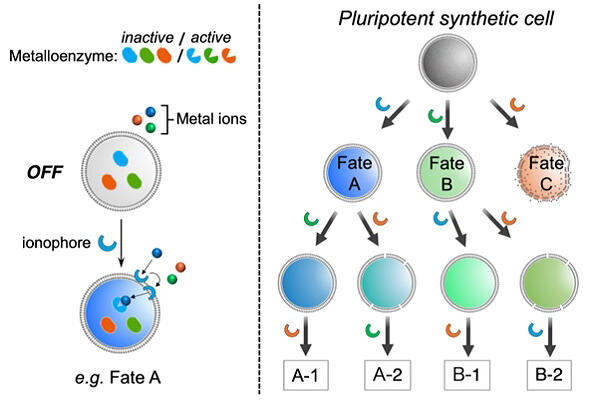
Right: Design of a pluripotent synthetic cell with selective metal ion transport. A synthetic pluripotent cell differentiates sequentially towards different fates, depending on the sequence in which three different ionophores (A, B and C) are added.
Provided by Gifu University
Research aiming to artificially create cells for the purpose of understanding cellular phenomena and applying them to biomaterials has been conducted in recent years. To mimic the functions of cells, researchers have developed artificial cells that exhibit important and fundamental functions, such as gene expression (replication, transcription, and translation), adenosine triphosphate (ATP) production, metabolism consisting of multiple enzyme reactions, cell division and fusion, and cell-to-cell adhesion and information transmission. Meanwhile, most existing artificial cells have been designed to exert function in response to external stimuli, focusing on one of these functions. Artificial cells that have multiple functions have not been developed successfully.
Previously, the research group developed giant liposomes (giant unilamellar vesicles [GUVs]) and used them as cell membranes to encapsulate biomolecules in research to develop artificial cells. In this study, the research group aimed to mimic the adaptability of cells with artificial cells and develop artificial cells that respond to three different stimuli. In cells, the mechanisms to correctly deliver external stimuli to the inside are important. Various reactions (cellular phenomena) occur after membrane proteins, such as transporters, channels, and receptors expressed on the cell membrane, transport or bind to substances that cannot pass through the cell membrane.
Therefore, transporters that deliver specific stimuli to the inside were bound to artificial cell membranes, and three metal-dependent enzymes whose functions differ from each other were encapsulated within GUVs, which were cell-sized liposomes used as a cell membrane model. The activity of respective metalloenzymes was designed to be regulated through metal ion transport by ionophores (a group of molecules that increase the membrane permeability of specific metal ions) bound to the cell membrane.
A specific ionophore was bound to a cell membrane in an environment where three different metal ions coexisted outside the artificial cells. The artificial cells with the specific ionophore were shown to exert a function that differed depending on the ionophore selected. When the three different ionophores were attached to the cell membrane simultaneously, the transport of all metal ions was suppressed. Molecular dynamics simulations indicated that interactions between ionophores in the cell membrane would inhibit their movement within the cell membrane and prevent metal ion transport. Furthermore, when the three different ionophores were bound to the cell membrane in different orders, the activation level of a metalloenzyme corresponding to an ionophore that was later attached to the cell membrane was noticeably reduced. This result clearly indicates that the order of binding of the ionophores to the cell membrane affects the activation levels of the metalloenzymes.
Higashi said, "This study was mainly undertaken during my postdoctoral research in the Wegner laboratory at the University of Münster (Germany). I went through many hardships and learning experiences as I tried to develop an artificial cell model using giant liposomes, metalloenzymes, and ionophores. In particular, when encapsulating metalloenzymes in giant liposomes, it took more than half a year to find optimal conditions to remove metal ions for inactivating the enzymes. Moving forward, I hope to continue to develop functional artificial cells by utilizing diverse proteins involving metal ions."
Journal Information
Publication: Nature Chemistry
Title: Adaptive metal ion transport and metalloregulation-driven differentiation in pluripotent synthetic cells
DOI: 10.1038/s41557-024-01682-y
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




