An international joint research group led by Professor Masatoshi Kudo of the Department of Gastroenterology and Hepatology at the Kindai University Faculty of Medicine, has announced that the conventional transarterial chemoembolization (TACE) combined with two drugs, lenvatinib (a molecular-targeted agent) and pembrolizumab (an immune checkpoint inhibitor), showed an improved therapeutic efficacy than the conventional method in patients with unresectable, nonmetastatic hepatocellular carcinoma. In a multicenter, randomized, double-blind, phase III study, the researchers confirmed that the combination significantly prolonged progression-free survival and prolonged overall survival. The combination is expected to be applied to standard care. The results were published in the January 8 issue of the international journal The Lancet.
Provided by Kindai University
Hepatocellular carcinoma is a poor-prognosis cancer with a high recurrence rate of approximately 70% within two years after resection. Moreover, hepatocellular carcinoma is often accompanied by liver dysfunction such as chronic hepatitis and is considered unresectable in such cases. TACE is a therapeutic procedure to kill cancer cells by injecting an anticancer agent into the lesion and an embolization agent to block blood vessels and it is indicated for unresectable hepatocellular carcinoma. The procedure was developed in Japan. After sorafenib (a molecular-targeted agent) was developed in 2009, a series of therapeutic agents have been developed, including lenvatinib, developed in 2018. Six clinical trials have been conducted to test combinations of TACE with these drugs to improve the efficacy; however, all but one trial by this research group failed. Recently, anti-PD-L1 antibody drugs have been developed, and immunotherapy with these drugs is now a first-line treatment.
In a previous investigator-initiated trial of lenvatinib combined with TACE (LEN-TACE therapy), the research group confirmed that this combination showed a favorable therapeutic effect. They also found that long-term administration of lenvatinib prior to TACE improved the therapeutic efficacy of this combination through normalization of tumor blood vessels and other effects.
In the present study, the research group conducted a multicenter, randomized, double-blind, phase III trial to evaluate the efficacy and safety of LEN-TACE combined with pembrolizumab (an immune checkpoint inhibitor) in patients with unresectable, nonmetastatic hepatocellular carcinoma and examine whether the therapeutic efficacy can be improved further. Of patients with conditions for which TACE was indicated from 137 sites in 33 countries or regions, 480 patients who met the criteria were included in the study. Patients were randomized to the TACE + lenvatinib + pembrolizumab group or the TACE + placebo group (placebo group), and the lengths of progression-free survival and overall survival after treatment were investigated.
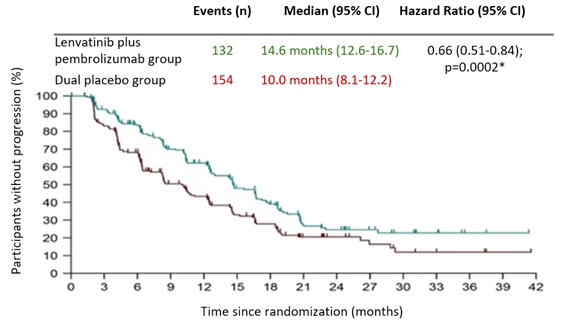
While the median progression-free survival was 10 months in the placebo group, it was extended to 14.6 months in the TACE + lenvatinib + pembrolizumab group. The 24-month overall survival rates in the TACE + lenvatinib + pembrolizumab group and the placebo group were 75% and 69%, respectively. In the clinical trial, tumors were reduced in size or disappeared in some patients.
Journal Information
Publication: The Lancet
Title: Transarterial chemoembolisation combined with lenvatinib plus pembrolizumab versus dual placebo for unresectable, non-metastatic hepatocellular carcinoma (LEAP-012): a multicentre, randomised, double-blind, phase 3 study
DOI: 10.1016/S0140-6736(24)02575-3
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




