A research group led by Specially Appointed Assistant Professor Christian Ganser and Specially Appointed Researcher Shigetaka Nishiguchi (currently Specially Appointed Assistant Professor at the Graduate School of Engineering, Osaka University) at the Exploratory Research Center on Life and Living Systems (ExCELLS), National Institutes of Natural Sciences, Researcher Feng-Yueh Chan and Professor Takayuki Uchihashi (also a visiting professor at ExCELLS) at the Graduate School of Science, Nagoya University, is the first in the world to successfully develop a new microscopy technique "high-speed in-line force mapping (HS-iFM)" to measure the dynamic nanoscale mechanical properties of the membrane of living bacteria. They revealed how the stiffness of the E. coli cell membrane changes during cell division. The findings were published in Science Advances.
Provided by Nagoya University
Previous studies have indicated that drug administration and malignant transformation induce changes in cell stiffness. The development of microscopy techniques to measure such changes with nanometer-level resolution has been awaited. However, optical microscopy has a limitation in its ability to observe small structures, and electron microscopy cannot observe biological samples in the living state. Meanwhile, atomic force microscopy (AFM) can measure the mechanical properties of materials at high resolution, but conventional AFM is unsuitable for observing dynamic phenomena occurring in living cells because of the slow imaging speed.
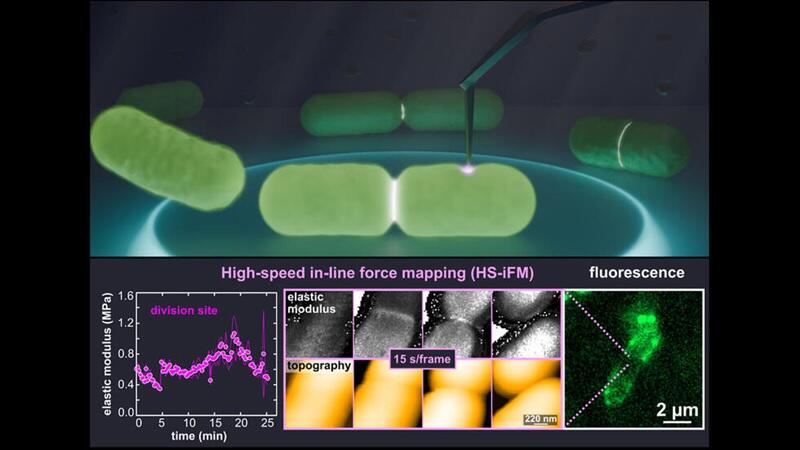
The research group developed HS-iFM, which combines an increased AFM scanning speed with mechanical property measurements. This technique realized simultaneous measurement of molecular-level surface topography and mechanical properties of living cells. Observation of E. coli cells undergoing cell division using HS-iFM showed that the membrane at the division site stiffened markedly compared with the surrounding area. The stiffening is attributed to local increases in membrane tension and cell wall thickness. Ganser commented, "By observing living bacteria, now we can directly follow their changes over the course of their life activities. Although E. coli has been well studied, its dynamic nanoscale mechanical changes have mostly remained a mystery."
They also observed the formation and eventual rupture of a membrane bridge connecting the two daughter cells during cell division. The formation and rupture of such a bridge took 242 seconds on average, and this process was observed seven times. Soft regions less than 100 nanometers in diameter were observed in dividing cells, and their rupturing caused cell depressurization and death. In dividing cells that were not completely separated, one daughter cell underwent depressurization when the adjacent daughter cell ruptured.
Furthermore, they observed the formation, closure, and re-formation of pores on the cell membrane of E. coli during division. These pores are considered to be related to the formation of membrane vesicles (outer membrane vesicles) and are known to occur at an increased frequency around the new cell walls during cell division. However, the diameter of the observed pores (34.7 nanometers) was larger than previously reported values for membrane protein complexes (approximately 8 nanometers), and further investigation is necessary.
The HS-iFM technique has the potential to advance the research on a wide range of biological samples. Ganser said, "Moving forward, I would like to study the local and dynamic effects of external stimuli, such as antibiotics, on the nanomechanical properties of the membrane of living bacteria." The research team will further increase the speed and resolution of HS-iFM, aiming to visually observe the mechanical properties of small molecules, such as individual proteins.
Journal Information
Publication: Science Advances
Title: A look beyond topography: Transient phenomena of Escherichia coli cell division captured with high-speed in-line force mapping
DOI: 10.1126/sciadv.ads3010
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




