A research group led by Assistant Professor Yuto Yoshinari and Professor Takashi Nishimura of the Institute for Molecular and Cellular Regulation at Gunma University, Professor Ryusuke Niwa of the Life Science Center for Survival Dynamics, Tsukuba Advanced Research Alliance at the University of Tsukuba, and Professor Taishi Yoshii of the Graduate School of Environmental, Life, Natural Science and Technology at Okayama University, has announced that they elucidated part of the mechanism that prevents excessive protein intake using a model organism, Drosophila melanogaster. By inhibiting the function of each of nine intestinal hormones, they found that the intestinal hormone "CCHa1" suppresses the appetite for proteins. The findings are expected to find application in treatment targeting intestinal hormones. The results were published in the international journal Nature Communications on December 30.
Living organisms are understood to monitor the nutrients they consume and maintain nutritional balance by selecting foods that compensate for any deficiencies. Drosophila melanogaster makes food choices based on the nutritional status in their bodies, much like humans and other mammals. For example, individuals fed only on sugar or females that need to lay many eggs selectively consume protein-rich foods.
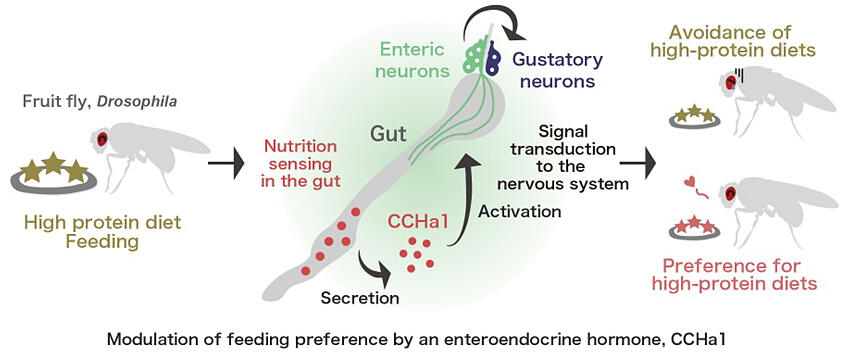
Recent studies using mammals and Drosophila species have revealed that enteroendocrine cells fire in response to dietary nutrients, releasing intestinal hormones to regulate metabolic balance according to food intake. However, it remains unclear whether these cells also control appetite for specific nutrients (dietary preferences). In this study, the research team explored whether enteroendocrine cells regulate dietary preferences.
First, to identify the subset of the enteroendocrine cells involved in feeding behavior in D. melanogaster, they inhibited the function of each of nine enteroendocrine hormones to observe effects of inhibition on feeding behavior. The results showed that functional inhibition of the enteroendocrine hormone CCHa1 increased food intake. When examining whether appetite increased for carbohydrates or proteins, they found that inhibiting CCHa1 led to excessive protein intake.
Examination of the activation of enteroendocrine cells producing CCHa1 showed that they were activated by a high-protein diet and the nonessential amino acids alanine and glycine. They also found that CCHa1 detects the protein content in food and functions to regulate amino acid intake. Moreover, an examination of the CCHa1 receptor revealed that the receptor, which is expressed in neurons from the brain to the gut, prevents excessive protein intake. Furthermore, the researchers examined the effects of disrupted CCHa1-mediated feeding regulation on the organism. They found that Drosophila with inhibited CCHa1 function had a shorter lifespan when raised on a high-protein diet.
To clarify the reason for the shortened lifespan, the research group comprehensively measured metabolites and found that intermediate metabolites in the urea cycle, which is important for the detoxification and excretion of amino acid-derived ammonia, were increased, leading to the accumulation of ammonia. Both Drosophila and humans obtain approximately 15% of their caloric intake from proteins. In humans, some enteroendocrine cells are known to respond to protein feeding, and enteroendocrine hormones may regulate appetite for proteins.
Yoshinari said, "It was challenging to determine which amino acids induce gut hormones. Moving forward, we aim to uncover what dictates dietary preferences for protein, such as omnivorous or carnivorous eating habits."
Journal Information
Publication: Nature Communications
Title: A high-protein diet-responsive gut hormone regulates behavioral and metabolic optimization in Drosophila melanogaster
DOI: 10.1038/s41467-024-55050-y
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




