On January 17, Lecturer Daisuke Aki of the Institute of Advanced Medicine, School of Medicine at Wakayama Medical University (Keio University School of Medicine at the time of research), in collaboration with Keio University and Tokyo University of Science, has announced that liver inflammation in metabolic dysfunction-associated steatohepatitis (MASH) is suppressed by increased regulatory T cells. Analysis of MASH model mice revealed that gene defects of the T cell-specific nuclear receptors of the Nr4a family noticeably increased regulatory T cells and enhanced their anti-inflammatory function. These findings are expected to contribute to the development of a therapeutic approach for MASH. The results were published in the international journal The Journal of Clinical Investigation.
Provided by Wakayama Medical University
MASH refers to metabolic dysfunction-associated steatohepatitis, which has previously been known as NASH. MASH arises from non-alcoholic fatty liver disease and may develop into chronic hepatitis, hepatic cirrhosis, and then hepatic cancer at advanced stages. MASH is on the rise worldwide, with more than three million people affected in Japan alone; however, no effective treatment methods are available.
The liver contains various immune cells, including T cells, which enter through capillaries, alongside hepatocytes that primarily perform liver functions. In advanced stages of hepatitis, cell death triggers immune system activation, leading to fibrosis. This process, in turn, causes further cell death, creating a vicious cycle. As a result, a connection between immune cells and MASH has been suspected, but details remained unclear.
Previous research by Professor Akihiko Yoshimura and his colleagues at the Research Institute for Biomedical Sciences at Tokyo University of Science (Keio University at the time of their research) has shown that the nuclear receptor Nr4a family is involved in the differentiation of T cells into regulatory T cells (Tregs), CD8 T cells, and other cells and serves as a brake for immune responses. Upon binding to extracellular substances such as hormones, nuclear receptors are translocated into the nucleus, where they bind to specific gene sequences and regulate transcription.
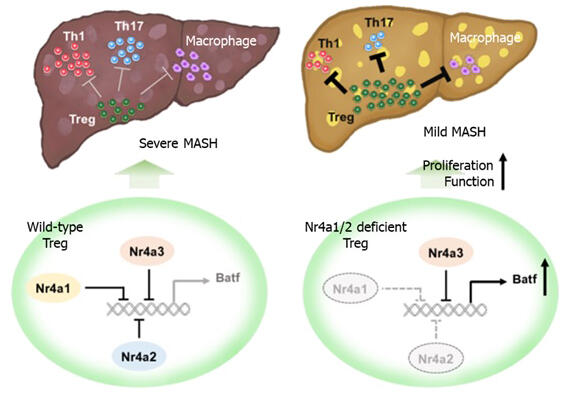
In this study, the group analyzed Nr4a gene expression in liver T cells of mice with diet-induced MASH to clarify the relationship between T cells and MASH. They found that Nr4a expression levels in CD4 and CD8 T cells were higher in MASH model mice than in wild-type mice. The Nr4a family comprises Nr4a1 to 3, which are very similar molecules. In mice, the complete loss of Nr4a genes in T cells is fatal due to severe autoimmune disease. Therefore, the researchers induced MASH in mice lacking both Nr4a1 and Nr4a2 (dKO mice).
Consequently, characteristics of MASH, such as reduced liver function, liver cell death, inflammatory macrophage infiltration, and liver fibrosis, were suppressed in dKO mice. Moreover, CD8 T cell-specific Nr4a1/2 defects did not impart MASH resistance to mice, indicating that Nr4a in CD4 T cells is involved in the pathogenesis of MASH.
Next, to clarify this mechanism, they examined the distribution of T cells in the liver and found that Th1 and Th17 cells, which promote inflammatory responses, were reduced in the livers of dKO mice with induced MASH, whereas Tregs, which suppress inflammatory responses, were markedly increased. Furthermore, single-cell RNA sequencing of CD4 T cells isolated from wild-type and dKO mice was analyzed. The analysis revealed that only in CD4 T cells from the liver of dKO mice, a specific Treg subset highly expressing IL10, which suppresses immune responses, was increased, and the IL10 expression was also increased. They also confirmed that the transcription factor Batf was strongly expressed in this specific subset of Tregs, and the strong Batf expression was suppressed by forced expression of Nr4a2. Moreover, they confirmed that suppression of Batf expression in Tregs from dKO mice resulted in reduced cell proliferation and IL10 production. Moving forward, the research team is going to develop methods for culturing and increasing the desired subset of regulatory T cells.
Aki said, "Our results suggest that two things can be expected. First, increasing Tregs may be an effective prophylactic and therapeutic strategy for MASH. Second, if a method to moderately suppress Nr4a or increase Batf expression in T cells can be developed, it will be possible to amplify Tregs that are very effective against inflammation. We hope that T-cell-targeted therapy for MASH can be developed by combining these approaches."
Journal Information
Publication: The Journal of Clinical Investigation
Title: The Nr4a family regulates intrahepatic Treg proliferation and liver fibrosis in MASLD models
DOI: 10.1172/JCI175305
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




