A research group at the Osaka Institute of Technology succeeded in developing soap bubbles called gas marbles, which are stable in basic environments but break when exposed to acid, releasing the gas inside. Bubbles are formed by adsorbing polymer particles onto a water film instead of a surfactant. Using this technique, they created soap bubbles using cinnamon particles. These bubbles can be used in cakes and other foods to enjoy the texture and sound when biting into them and are expected to be applied to cosmetic fragrances and other products.
A research group led by Professor Syuji Fujii, who studies interfacial colloid chemistry and polymer chemistry at the Department of Applied Chemistry at Osaka Institute of Technology, has been investigating various bubbles and found that their stability changes depending on conditions such as pH and temperature, which represent hydrogen-ion concentrations.
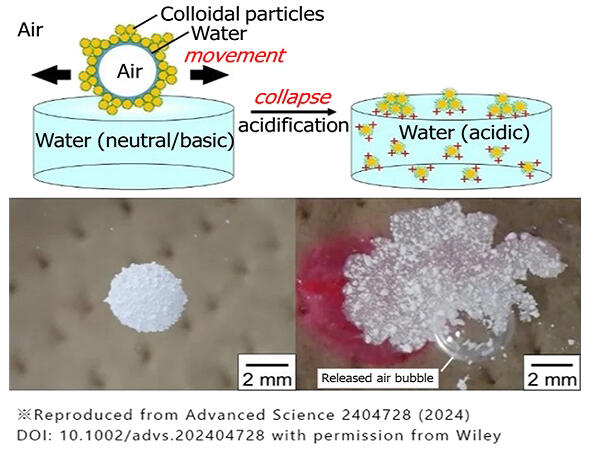
In this context, they have been working on a way to stabilize soap bubbles with fine particles. Soap bubbles are generally stabilized using surfactant molecules found in detergents, but this is an issue because they can cause allergic reactions. Therefore, Fujii came up with the idea of covering the surface of a water film of soap bubbles with special basic polystyrene particles, which are polymer particles whose compatibility with water can be controlled using pH.
Provided by Osaka Institute of Technology
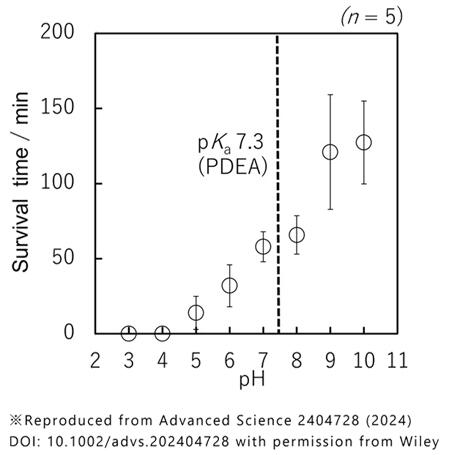
Initially, a petri dish was filled with water, and dry polystyrene particles were sprinkled on it, covering the water surface with particles. Then, air bubbles were pumped into the water, similar to in a fish tank, and the bubbles which were covered with particles, rose to the surface. When bubbles were rolled over the water surface covered with particles, a spherical gas marble was formed. These polystyrene particles exhibited a property of having the balance between hydrophilic and hydrophobic properties on their surface, which can be controlled by pH. Gas marbles cracked instantly and released internal air when the pH was 3-4 (acidic) but could remain stable for approximately 2 h when pH was 9-10 (alkaline).
Provided by Professor Syuji Fujii of Osaka Institute of Technology
The surface structure of the created gas marbles was observed using an electron microscope, which showed that some polystyrene particles were aggregated, while others were stuck together in a single layer; additionally, the surface was uneven. The film of particles covering the gas marble makes it "amphibious," allowing them to roll on the surface of water and on solids.
Fujii believed that this mechanism could be applied to flavoring food and began to work on creating edible gas marbles. Using cinnamon particles as a stabilizer, gas marbles maintained their spherical shape without cracking as water evaporated, and they were successfully stored for a long time. When made with milk, soy milk, or coffee instead of water as the liquid, they retained their spherical shape for a long period of time. In particular, gas marbles made from milk were very sturdy and gave a crunchy sound when chewed with the teeth, and one can enjoy the delicious aroma and texture of cinnamon.

Provided by Osaka Institute of Technology
Fujii said, "Because air does not conduct heat satisfactorily, it can be used as an insulation material. We want to continue to explore the types of particles that can be used to stabilize gas marbles. In the future, we want to develop a method by which gas marbles can be broken by changes in temperature, light, and pressure." In addition to food products, the group are also considering practical applications in cosmetics and hair care products.
Both studies were supported by a grant-in-aid for scientific research from the Japan Society for the Promotion of Science (JSPS). The results of the study on acidic soap bubbles were published in the electronic edition of the German scientific journal Advanced Science and later announced by the Osaka Institute of Technology. The results of the study on cinnamon bubbles were published in the electronic edition of the German scientific journal Advanced Functional Materials and then announced by the University.
Original article was provided by the Science Portal and has been translated by Science Japan.




