Alzheimer's disease and Parkinson's disease develop as a result of amyloid fibril formation by the respective causative proteins. Certain stimuli are thought to trigger amyloid formation, but it has remained unclear what they are exactly. A research group led by Specially Appointed Researcher Yuji Goto, Doctoral Student Tomoki Ota, and Professor Hirotsugu Ogi of the Graduate School of Engineering at the University of Osaka, and Professor Suguru Yamamoto of the Graduate School of Medical and Dental Sciences at Niigata University has announced their research results showing that "shear stresses" caused by peristaltic pumps, which pump liquids by squeezing tubes with rollers, trigger the formation of a substance called the amyloid fibril. The findings are expected to facilitate the evaluation of amyloidogenic risks and other amyloid-related assessments. The study was published in npj Biosensing.
Provided by the University of Osaka
Amyloidosis is a general term for diseases caused by the accumulation of amyloid fibrils formed by causative proteins. More than 30 different forms of amyloidosis are known, including Alzheimer's disease, Parkinson's disease, and dialysis-related amyloidosis. Previous studies have shown that amyloid fibrils have a crystal-like structure and are formed when an excessive denatured protein precipitates upon certain stimuli. The stimuli used for in vitro amyloid formation include agitation and ultrasonication of the solution; however, the in vivo triggers have remained unknown.
While investigating amyloid formation reactions dependent on triggers, such as ultrasonication and solution agitation, the research group noticed that the model protein hen egg white lysozyme formed amyloid fibrils merely by passing the solution through a peristaltic pump. Furthermore, they confirmed that the peristaltic pumps also trigger the formation of amyloid fibrils from other amyloidogenic proteins. Such amyloidogenic proteins include α-synuclein responsible for Parkinson's disease, amyloid β responsible for Alzheimer's disease, and β2-microglobulin responsible for dialysis-related amyloidosis.
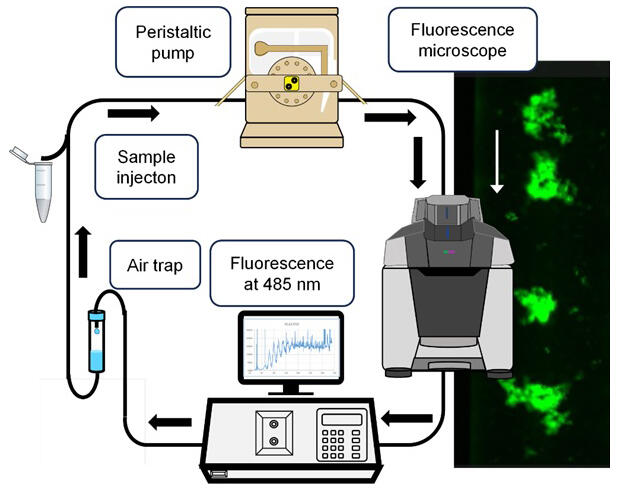
A looped system combining a peristaltic pump, a thioflavin T fluorescence detector, and a fluorescence microscope to observe thioflavin T-stained aggregates was used to monitor the temporal changes in amyloid formation, the morphology of amyloid aggregates, and the change in amyloid morphology in real-time. The paper includes movie files showing these observation data.
For all proteins, they observed that amyloid fibrils formed clumps with fluff-like morphology and the clumps changed their morphology while they were carried by a laminar flow in a channel 1 mm in diameter. The amyloid fibrils of amyloid β looked particularly sticky, forming even larger clumps on their own and adsorbing to the observation cell. Amyloid precursors of β2-microglobulin looked like a firefly flashing.
Finite element method (FEM) analysis suggested that the peristalsis-caused shear stress was 10,000 times higher than that produced by a typical laminar flow. They also successfully simulated the growth and dilution of amyloid fibrils circulating in the looped system through model calculations. Their simulations showed that the peristaltic motion of the peristaltic pump can produce particularly strong shear stress.
Amyloid formation induced by extremely high shear stresses produced by the peristaltic pump, which is a general-purpose device, was a completely unexpected finding. The possibility of peristaltic pump-triggered amyloid formation is a completely new discovery and warrants careful examination to ensure that peristaltic pumps used in medical practice do not pose such risks. Many flow channels in the body are subject to strong shear stress, including microvessels and lymphatic vessels.
In the present study, such flow channels were suggested to be the initiation sites of amyloid formation. These results are expected to greatly advance our understanding of the mechanism of amyloid formation in vivo and contribute to the prevention and treatment of amyloidosis.
Goto said, "The video of amyloid flow revealed through the microscopic observation in this study reminded me of a scene of 'white-water rafting' in the 1954 movie 'River of No Return,' starring Marilyn Monroe. Thus, I named the amyloid flow the 'Amyloid River of No Return.' 'River of No Return' concluded happily. I hope that 'Amyloid River of No Return' will also come to a happy ending through the efforts of researchers."
Journal Information
Publication: npj Biosensing
Title: Peristaltic pump-triggered amyloid formation suggests shear stresses are in vivo risks for amyloid nucleation
DOI: 10.1038/s44328-025-00027-0
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




