To achieve carbon neutrality in the chemical industry, efforts are underway to use raw materials other than fossil resources. In particular, the electrochemical CO2 reduction reaction (CO2RR), which directly converts CO2 into chemical raw materials using electricity derived from renewable energy sources, has attracted attention as a radical method for decreasing the CO2 concentration in the atmosphere. However, the energy efficiency of CO2RR is currently low, and increasing this efficiency has been a major challenge for its practical applications.
A research group including Dr. Takaaki Tomai, Professor of the Frontier Research Institute for Interdisciplinary Sciences at Tohoku University, increased the efficiency of the CO2RR process utilizing a high-temperature, high-pressure water environment (hydrothermal reaction field). In general, industrial water electrolysis is performed at high temperatures of up to 100℃ because electrochemical reactions accelerate at higher temperatures. However, CO2 solubility in water and energy efficiency of the CO2RR process decrease under excessively high-temperature conditions. The research group overcame this problem by using a high-pressure environment of 100 atm and higher. They demonstrated that a hydrothermal environment of 150℃ temperature and 100-atm pressure notably increases the energy efficiency of the CO2RR process by increasing CO2 supply to the electrodes via increasing CO2 solubility and diffusion coefficient value.
The research group also focused on the use of unused low-temperature waste heat released from factories and power plants as an energy source to create a high-temperature environment. Through a technology assessment, they also demonstrated the possibility of "carbon-negative" methanol production, in which CO2 absorption exceeded its emission. This result, achieved by incorporating a chemical engineering approach into the electrochemical process, will drive the chemical industry toward realizing a circulation society in which CO2 is converted into a highly efficient resource. Electrolysis technology utilizing hydrothermal reaction fields will be important in the next-generation sustainable, recycling-oriented society.
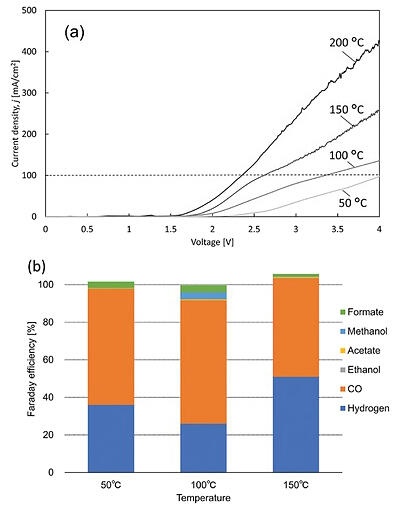
(b) Product percentage at various temperatures up to a current density of 100 mA per square centimeter. As current density increases with temperature, the same reaction proceeds at lower voltages, resulting in increased energy efficiency.




