A research team led by Professor Kenji Matsuda, Senior Lecturer Kenji Higashiguchi, and Graduate Students Kurumi Satake and Naoto Ootsuki (at the time of the research) of the Department of Synthetic Chemistry and Biological Chemistry, Graduate School of Engineering at Kyoto University, has successfully synthesized a double-closed form of a diarylethene fused dimer where both units are cyclized through light and electrochemical stimulation. They demonstrated that this compound could undergo stepwise switching between three states that absorb ultraviolet, visible, and near-infrared light respectively.
Matsuda commented, "Until now, diarylethene fused dimers were expected to be near-infrared absorbing responsive materials, but they were dream compounds that could not be synthesized. Even after starting research with the idea of using light and electrochemistry, it took more than five years. The reaction mechanism, where the first step only proceeds with light and the second step only with electrochemistry, exceeded our initial expectations, and I felt the power of compounds found in nature. I hope this will lead to an expansion of the world of functional molecular materials combining light and electrochemistry."
Their findings were published in the Journal of the American Chemical Society.
Provided by Kyoto University.
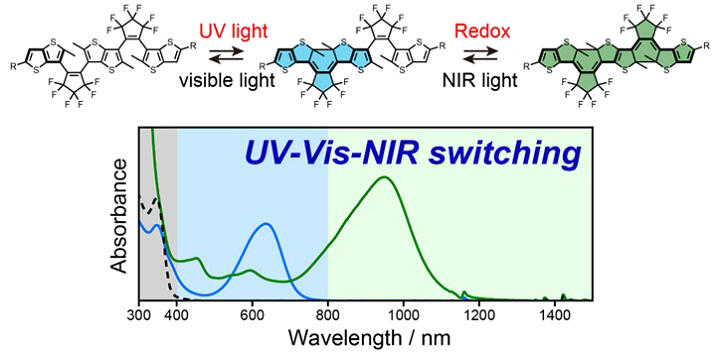
Diarylethene is a type of organic dye that has the property of coloring or decoloring with light. The original molecular structure is an open-ring form, a colorless transparent molecule that cannot absorb visible light. When it absorbs ultraviolet light, the molecular structure changes to a closed-ring isomer. At this time, the π-conjugated structure in the molecule becomes longer, enabling it to absorb visible light, which results in coloration.
If two diarylethenes could be connected in a closed-ring state, the π-conjugated structure in the molecule would become even longer, allowing it to absorb near-infrared light. However, it is not possible to obtain such a double closed-ring isomer through a photoreaction. After using ultraviolet light to close one ring, attempting to close the other ring fails because the energy escapes to the side that first became a closed-ring isomer. This reactivity issue could be resolved by weakening the π-conjugation connection between the two closed rings, for example, by inserting a spacer that is unrelated to the reaction or by making the structure flexible rather than ladder-type so that the π-conjugation can move flexibly. However, with such flexible structures, the π-conjugation does not fully connect, and the absorption wavelength does not reach the near-infrared region. Therefore, the research group successfully closed both diarylethene units by combining the conventional photochromic ring-closing reaction with an electrochemical ring-closing reaction.
They synthesized a diarylethene fused dimer with appropriate substituents and investigated the structural changes accompanying light and electrochemical stimulation. First, upon irradiation with ultraviolet light, the colorless open-ring isomer changed to a light blue mono-closed isomer. Subsequently, by performing oxidation and reduction in sequence (applying electrochemical stimulation), a double closed-ring isomer with absorption in the near-infrared region was obtained. The structure was confirmed by single-crystal X-ray structural analysis.
By examining the structural changes accompanying the second-stage oxidation-reduction in detail and supporting this with quantum chemical calculations, they revealed that the electrochemical ring-closing reaction proceeded via a mechanism called radical coupling. Furthermore, it was confirmed that the double closed-ring isomer returned to the original mono-closed form upon irradiation with near-infrared light and then returned to the open-ring form upon irradiation with visible light. In other words, they found that the synthesized diarylethene fused dimer is a molecule capable of stepwise and reversible switching between three states that absorb ultraviolet, visible, and near-infrared light.
Matsuda commented, "It has been known for some time that diarylethenes react not only to light but also to oxidation-reduction. However, since almost no advantages of electrochemistry over light or no phenomena that can only be induced by electrochemistry were found, light alone was considered sufficient, and the value of electrochemistry was overlooked. I believe this discovery will expand the chemical space for many photofunctional materials, not limited to diarylethenes."
Journal Information
Publication: Journal of the American Chemical Society
Title: NIR-Responsive Double Closed-Ring Isomer of a Diarylethene Fused Dimer Synthesized by Stepwise Photochemical and Oxidative Cyclization Reaction
DOI: 10.1021/jacs.4c17757
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




