A research group led by Professor Hiromitsu Maeda from the College of Life Sciences at Ritsumeikan University, in collaboration with Kyoto University, Kitasato University, Tohoku University, the National Institute for Materials Science, Ehime University, and Shinshu University, has announced the successful synthesis of aπ-electronic cation, benzoporphyrin AuIII complex. They achieved the formation of pseudo-polymorphic assemblies and electrical conductivity through combinations with bulky counteranions. This approach is expected to be applied to the creation of materials based on charged π-electron systems. Their results were published in the Royal Society of Chemistry journal Chemical Science on February 19.
Provided by Ritsumeikan University
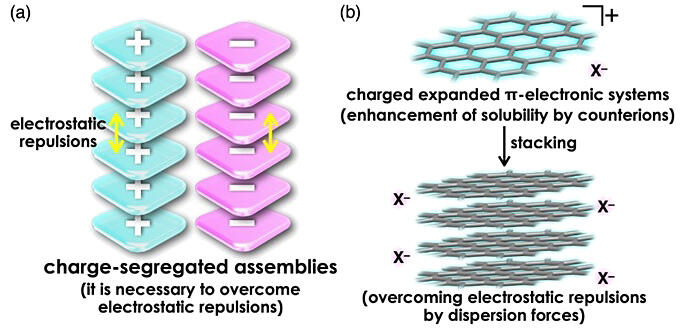
Assemblies consisting of stacked structures of π-electron systems with the same charge are expected to exhibit electrical conductivity. However, electrostatic repulsion between same-charge species hinders stacking, making it difficult to elucidate the assembly, structure, and functions. Generally, π-electron system molecules with expanded planar structures have low solubility, which has been a barrier to material formation and processing.
Previously, the research group had succeeded in stacking π-electron systems with the same charge using dipole-dipole interactions, revealing that utilizing intermolecular interactions is important for assembly. In this study, they newly synthesized a highly soluble ion pair by introducing appropriate counteranions to benzoporphyrin AuIII complex (BPAu+) as an expanded planar π-electronic cation. Due to the bulky counteranions, this ion pair showed high solubility in common organic solvents. The group also found that the stacked structure was formed along the long axis of the single crystal.
Using single-crystal X-ray structural analysis, they revealed that the ion pair forms charge-segregated assemblies in the crystalline state, where expanded π-electronic cations are stably stacked at a distance of 3.29 Å. Furthermore, interaction energy decomposition analysis using theoretical calculations showed that dispersion forces greatly contribute to the stacking of expanded π-electronic cations, overcoming electrostatic repulsion.
The BPAu+-FABA− ion pair also achieved pseudo-polymorphic assembly from a low-crystallinity state under solvent conditions different from single crystal preparation. Structural analysis revealed that it forms a hexagonal columnar structure based on the stacked structure of expanded π-electronic cations, with the cations stacked at a distance of 3.4 Å. These charge-segregated assemblies were also confirmed to exhibit electrical conductivity attributed to the stacked structure of the π-electronic cations.
It is common to introduce soluble substituents to add solubility to poorly soluble π-electron systems. In this case, the group achieved improved solubility by the introduction of a charge and selection of the shape of coexisting counter ions. Furthermore, they developed a method to form charge-segregated assemblies that overcome electrostatic repulsion by utilizing the dispersion forces acting on expanded π-electron systems.
Maeda commented, "The fact that introducing opposite charges to differently shaped molecular structures dramatically improves the solubility of highly planar π-electron system molecules, resulting in various assembly forms such as crystalline and non-crystalline, was something we did not anticipate at the beginning of the research. While the design and synthesis of cations and anions as constituent elements are fundamental to ion pair creation, this leads to the creation of new functional materials where the properties of ions with different charges work synergistically."
Journal Information
Publication: Chemical Science
Title: Electrically conductive charge-segregated pseudo-polymorphs comprising highly planar expanded π-electronic cations
DOI: 10.1039/d4sc07576e
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




