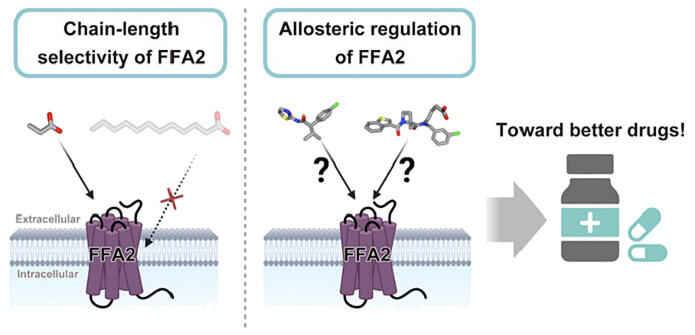
Short-chain fatty acids, which play an important role in maintaining health, are produced when dietary fiber is broken down by intestinal bacteria. These short-chain fatty acids trigger various physiological effects, such as regulation of metabolism and immunity, through the short-chain fatty acid receptor (FFA2) present on the cell membranes of the intestine, adipose tissue, pancreas, and immune cells. In recent years, FFA2 has attracted significant attention as a therapeutic target for lifestyle-related diseases and inflammatory bowel disease, with several candidate therapeutic compounds already developed and some advancing to clinical trials. However, it remained unclear how FFA2 selectively recognizes short-chain fatty acids and how these drugs targeting FFA2 regulate its function.
A research group including Graduate Student Mai Kugawa of the Department of Biological Sciences, Graduate School of Science, Research Fellow Kouki Kawakami and Professor Hideaki Kato of the Research Center for Advanced Science and Technology at the University of Tokyo, and Assistant Professor Ryoji Kise and Professor Asuka Inoue of the Graduate School of Pharmaceutical Sciences at Tohoku University has elucidated the mechanism by which FFA2 selectively recognizes short-chain fatty acids. Also, the research group revealed that FFA2 function is regulated by a new activation and blocking mechanism. Their research was published in Nature Communications.
Provided by the University of Tokyo
Using cryo-electron microscopy, the research group determined two types of three-dimensional structure: the activated form of FFA2 (with the trimeric G protein bound to the intracellular side) bound to two agonists (TUG-1375 and 4-CMTB) that activate FFA2, and the inactivated form of FFA2 bound to the antagonist GLPG0974, at resolutions of 3.19 Å and 3.36 Å, respectively. Based on these structures, they conducted molecular dynamics simulations and functional analysis experiments to investigate how FFA2 selectively recognizes short-chain fatty acids and how compounds targeting FFA2 regulate its function.
First, to elucidate the mechanism by which FFA2 selectively recognizes short-chain fatty acids, they compared the three-dimensional structure of FFA2 with that of the long-chain fatty acid receptor (FFA1). They found that FFA2 has a binding pocket for short-chain fatty acids with an entrance opening toward the extracellular side, while FFA1 has a binding pocket for long-chain fatty acids with an entrance opening toward the cell membrane. From this structural difference, they hypothesized that FFA2 can efficiently recognize highly water-soluble short-chain fatty acids but cannot recognize highly lipid-soluble long-chain fatty acids.
To test this hypothesis, they artificially created an entrance opening toward the cell membrane in FFA2 by replacing specific amino acids with different ones. As a result, the modified FFA2 with an entrance opening toward the cell membrane became capable of recognizing long-chain fatty acids, which it normally cannot recognize. This confirmed that FFA2 and FFA1 selectively recognize short-chain and long-chain fatty acids, respectively, due to the difference in the position of the entrance for free fatty acids.
Next, to understand the mechanism of action of the antagonist GLPG0974, they compared the three-dimensional structures of activated FFA2 and inactivated FFA2 bound to GLPG0974 and made an unexpected discovery. Previously, it was thought that GLPG0974 competitively inhibits the binding of short-chain fatty acids by binding to the same site as the site to which the short-chain fatty acids. However, they found that GLPG0974 actually binds to a novel site adjacent to the binding site of short-chain fatty acids. Furthermore, they revealed for the first time that when GLPG0974 binds to this site, a specific amino acid residue (Y90) is moved like a lever, distorting the shape of the binding site for short-chain fatty acids and indirectly inhibiting their binding, thus regulating FFA2 function—a mechanism not previously known for GPCR inhibitors. Finally, to investigate how the agonist 4-CMTB activates FFA2, they compared the three-dimensional structure of activated FFA2 bound to 4-CMTB with that of inactivated FFA2. It is known that the GPCR family, including FFA2, undergoes a significant structural change in the sixth and seventh transmembrane helices (TM) during activation. Surprisingly, they found that 4-CMTB binds to the outer surface between these two helices—an unexpected location. Normally, FFA2 without an agonist fluctuates between the activated and inactivated states, but 4-CMTB binds only when the receptor temporarily adopts an activated state and stabilizes that state, revealing for the first time a sophisticated mechanism of action not previously reported.
Kato commented, "FFA2 is an important membrane receptor that regulates metabolic and immune functions and has recently attracted attention as a gateway in communication between the intestinal microbiota and the host. The ligand recognition mechanism by FFA2 and the mechanism of FFA2 activity regulation by ligands that we have elucidated are unique and different from conventional activity regulation mechanisms. We expect that using this knowledge will accelerate drug discovery targeting FFA2 and similar membrane receptors in the future."
Journal Information
Publication: Nature Communications
Title: Structural insights into lipid chain-length selectivity and allosteric regulation of FFA2
DOI: 10.1038/s41467-025-57983-4
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




