A research team led by Principal Researcher Takeshi Kai at the Nuclear Science and Engineering Center of the Japan Atomic Energy Agency (JAEA), along with Professor Akinari Yokoya from the Graduate School of Science and Engineering at Ibaraki University, Associate Professor Yusuke Matsuya from the Faculty of Health Sciences at Hokkaido University, and Associate Professor Hidetsugu Tsuchida from the Graduate School of Engineering at Kyoto University, has revealed the mechanism of DNA damage that initiates radiation-induced cancer. Through simulation, they found that even at low doses, electrons and OH radicals generated from water molecules decomposed by radiation can simultaneously react with DNA, potentially causing complex DNA damage. This finding is expected to contribute to radiation protection measures. The results were published in the March 6 issue of the international academic journal Communications Chemistry.
Provided by JAEA
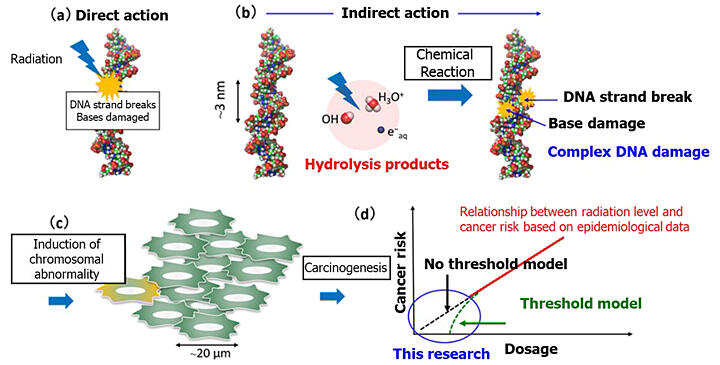
The risk of radiation-induced cancer has limited epidemiological data in the low-dose range (100 mSv) and is estimated based on models. Technology to experimentally detect DNA damage has not yet been realized. Meanwhile, there are models with and without thresholds for cancer-inducing doses, and it was unclear which model was correct.
DNA damage is an initial factor in cancer risk. When this damage is isolated, it is quickly repaired. However, when multiple damages (cluster damage) form within 10 base pairs, the effectiveness of damage site removal and repair by repair proteins decreases, as reported in experiments using synthetic DNA. This increases the risk of cancer.
The research group focused on indirect damage caused by reactions between radiation decomposition products generated near DNA and the DNA itself. They calculated the random diffusion motion of H3O+, OH radicals, and hydrated electrons in an aqueous solution containing DNA.
Assuming a single radiation beam hit a position x nm away from the DNA, with the spur radius of the ejected electron at α nm, they calculated the thermal diffusion motion of the generated decomposition products. They evaluated the chemical reactions between the generated decomposition products and DNA, enabling the assessment of isolated and clustered damage yields.
The results showed that when only OH radicals react with DNA, easily repairable isolated damage is formed, whereas when OH radicals and hydrated molecules react with DNA together, difficult-to-repair cluster damage is formed. In the case of cluster damage, when multiple decomposition products are generated by radiation energy transferred to water molecules in the very close vicinity of DNA (within 1.5 nm), cluster damage can form with a probability of less than 1% (about 1/50 the probability of isolated damage), potentially leading to cancer. According to the researchers, these results support a model without a threshold.
Kai stated, "In this study, we researched the so-called low-dose radiation effects, but we would like to extend this to high-dose areas such as cancer treatment as well."
Journal Information
Publication: Communications Chemistry
Title: Multiple DNA damages induced by water radiolysis demonstrated using a dynamic Monte Carlo code
DOI: 10.1038/s42004-025-01453-x
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




