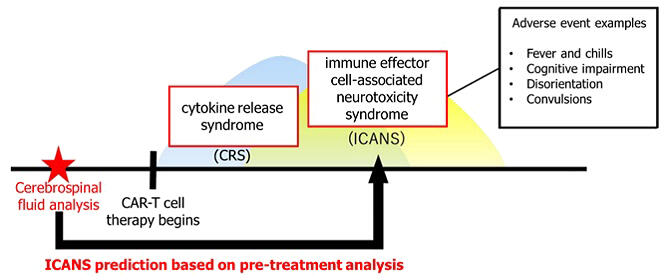
Chimeric antigen receptor T-cell (CAR-T) therapy is one of the highly effective cancer therapies. However, one problem of this therapy is immune effector cell-associated neurotoxicity syndrome (ICANS) as an adverse reaction, which can lead to neurological disorders. A research team led by Professor Yuya Kunisaki of the Department of Clinical Chemistry and Laboratory Medicine, Faculty of Medical Sciences at Kyushu University, Dr. Tomoko Nomiyama and Assistant Professor Daiki Setoyama of the Clinical Laboratory, and Associate Professor Koji Kato of the Department of Hematology, Oncology & Cardiovascular Medicine at Kyushu University Hospital, has successfully developed a biomarker to predict the risk of developing ICANS by testing spinal fluid proteins before treatment.
Kunisaki said, "We were able to detect changes in the profile of low-level proteins by utilizing the mass spectrometry platform in the Department of Clinical Laboratory. Moving forward, we will apply this technique to general biochemical assays and simple test kits. Our results also suggested that complement-targeting drugs may effectively prevent and treat ICANS, so we have begun efforts to develop prophylactic and therapeutic agents."
The results were published in the journal Leukemia.
Provided by Kyushu University
CAR-T therapy is an immunotherapy to treat cancer by returning a patient's T cells in which cancer cell-specific antibodies are incorporated into the body. This therapy is highly effective against malignant tumors such as B-cell non-Hodgkin's lymphoma.
The Japanese insurance system covers CAR-T therapy for the treatment of five types of cancer, including B-cell lymphoma, acute lymphocytic leukemia, and multiple myeloma. In Japan, CAR-T therapy is performed at 3 facilities in the Hokkaido and Tohoku regions, 17 in the Kanto region, 11 in the Kinki region, 8 in the Chubu region, 5 in the Chugoku and Shikoku regions, and 4 in the Kyushu and Okinawa regions. As of March 2023, CAR-T therapy was administered in 112 cases at Kyushu University Hospital. Because of its high response and complete remission rates, which were unachievable with conventional therapies, CAR-T therapy is one of the last-resort options in cancer treatment. However, the problems of this therapy include adverse effects, such as cytokine release syndrome and ICANS.
In particular, ICANS is a syndrome in the central nervous system that typically develops within a few days to a week after treatment. It manifests with serious symptoms in some cases, such as delirium, anxiety, dizziness, disturbance of consciousness, confusion, seizures, aphasia, and mutism. The severity varies from patient to patient, and some cases of sequelae have been reported. Its pathogenesis remains largely unknown, and it is difficult to control. Thus, predicting an early response to ICANS is key to improving the safety of CAR-T therapy. However, its onset has been difficult to predict.
In this study, the research group analyzed residual spinal fluid test specimens collected prior to CAR-T-cell therapy in 29 patients with B-cell non-Hodgkin's lymphoma. Kunisaki commented, "We selected a homogenous group of patients with no central nervous system mutations because we didn't want too many variables, which might hinder the identification of biomarkers."
Using the state-of-the-art mass spectrometry platform owned by the Department of Clinical Laboratory of Kyushu University Hospital, a comprehensive protein analysis was conducted to search for promising protein biomarkers. They screened the proteins detected in patients' spinal fluid specimens to identify those suitable to distinguish between patients who developed ICANS and those who did not. Among the approximately 1300 proteins in cerebrospinal fluid, they found the C1RL/FUCA2 ratio and the F12/FUCA2 ratio as biomarkers using a machine learning algorithm.
The receiver operating characteristic (ROC) curve analysis showed that the biomarkers achieved an area under the curve (AUC) of 0.95, which indicates a very high prediction accuracy. Similar results were obtained in 10 additional cases analyzed for confirmation. Complement-targeting drugs are candidate therapeutic agents for ICANS.
Kunisaki added, "Considering the biological pathways, the biomarkers found in this study may recapitulate the pre-inflammatory state. We are studying complement-targeting drugs that act on proteins involved in the immune responses as potential candidate drugs for preventing and treating ICANS."
Journal Information
Publication: Leukemia
Title: Cerebrospinal fluid proteomics exerts predictive potential for immune effector cell-associated neurotoxicity syndrome (ICANS) in CAR-T cell therapy
DOI: 10.1038/s41375-025-02541-6
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




