A research group from Hiroshima University has demonstrated that administering low-dose opioids to mice with autism spectrum disorder (ASD) improves their social behavior. Opioids, including morphine, are typically used at high doses to treat severe cancer pain. While there have been no medications specifically for ASD treatment, this research suggests the possibility of repurposing opioids appropriately as a therapeutic option.
Professor Yukio Ago, who specializes in central nervous system pharmacology, and his research group from the Department of Cellular and Molecular Pharmacology at Hiroshima University Graduate School of Biomedical and Health Sciences (Dentistry) have been investigating the relationship between opioid receptors and drugs that act on them through industry-academic collaboration with pharmaceutical companies.
Opioids include strictly regulated medical narcotic painkillers such as morphine, fentanyl, and oxycodone used for cancer pain, as well as buprenorphine, which is classified as a psychotropic drug. All these drugs strongly bind to µ (mu) opioid receptors in cell membranes, but buprenorphine is classified as a "partial agonist" with weaker activation of intracellular signaling compared to others. Opioids can also be endogenous, such as endorphins produced in the brain during times of stress.
Ago has been researching animal models of ASD. Since ASD involves a certain form of impairment of brain regions related to motivation maintenance and social communication, he hypothesized that drugs that effectively target these areas could potentially become treatments for ASD.
Provided by Hiroshima University
Opioid overuse and abuse have become social problems in many foreign countries, leading to addiction countermeasures. Dopamine, a neurotransmitter, is deeply involved in opioid addiction. When large amounts of agonists (chemicals/drugs that bind to and activate µ opioid receptors) are present, excessive dopamine release occurs, creating a risk for dependence.
On the other hand, when these agonists are appropriately regulated in suitable amounts, they can balance dopamine activity that governs motivation and drive. The researchers studied whether this could be applied to help with interpersonal relationship challenges faced by individuals with ASD.
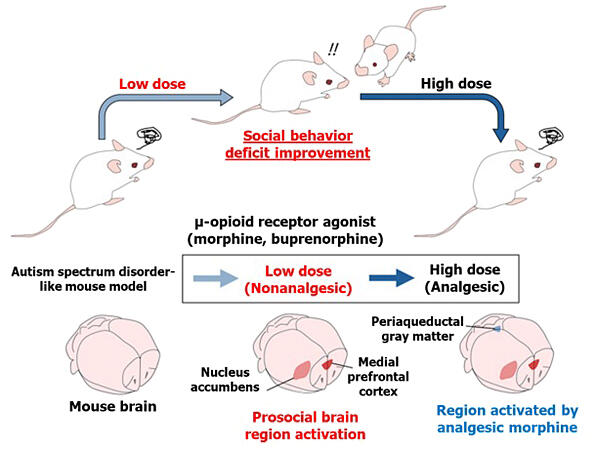
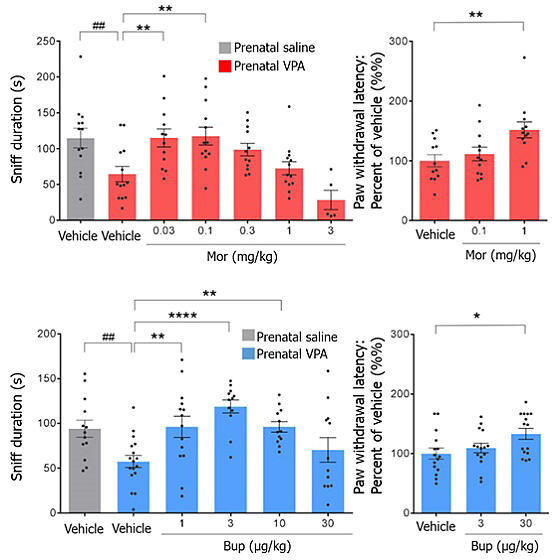
As a result, when low-dose opioids without pain-relieving effects were administered to ASD model mice, their sociability improved and they began interacting with other mice. As the dosage was increased to high-dose opioids with pain-relieving effects, the social improvement effects disappeared.
Provided by Hiroshima University
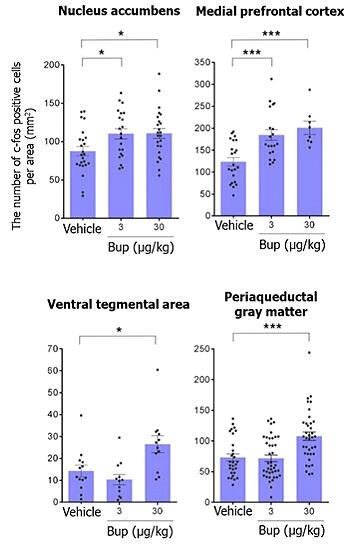
Furthermore, when investigating in detail which brain regions were activated with opioid administration using buprenorphine, the results showed that at low doses, regions involved in social behavior—the "medial prefrontal cortex" and "nucleus accumbens"—were activated.
On the other hand, at high doses, regions such as the "periaqueductal gray matter" and "ventral tegmental area" were also activated. The periaqueductal gray matter not only contributes to pain relief but also induces fear and discomfort, while the ventral tegmental area is involved in dependence formation. This indicates that high-dose opioids may have counterproductive effects for ASD. Buprenorphine was used in the research because it has a lower implementation barrier than morphine if it were to be put into practical use and because morphine is a narcotic drug.
Edited by the Science Portal Editorial Office and translated by Science Japan based on graphs provided by Hiroshima University
Ago and his team plan to use viral vectors to investigate whether the activation of these regions is essential for ASD treatment or merely a result of using the drugs, with other factors potentially involved, examining these subtle causal relationships.
This approach of repurposing drugs for conditions other than those they were originally intended for is called "drug repositioning." Previous examples include antihistamines being used as sleep aids due to their drowsiness side effects, and the sleep medication thalidomide becoming a multiple myeloma treatment because it inhibits new blood vessel formation. Low-dose opioids could potentially become another example of successful drug repositioning.
Ago notes challenges ahead in the practical use of these drugs: "It's difficult to directly utilize existing concentrated high-dose opioid formulations. We need to consider either reformulating existing painkillers at lower concentrations for optimization or creating new low-dose opioid compounds from scratch." He reflected that "low-dose opioids have extremely low dependency potential, making this an interesting discovery from a new perspective."
The research was conducted jointly by Hiroshima University, the University of Osaka, Kyoto University, and Shionogi Inc., with funding from the Japan Society for the Promotion of Science's Grants-in-Aid for Scientific Research, the Nakatomi Foundation, the Astellas Foundation for Research on Metabolic Disorders, and the Mochida Memorial Foundation for Medical and Pharmaceutical Research. The findings were published in the electronic version of the American scientific journal JCI Insight on December 6, 2024, and jointly announced by Hiroshima University and the University of Osaka on February 10, 2025.
Original article was provided by the Science Portal and has been translated by Science Japan.




