A research group led by Clinical Associate Kenji Matsui, Professor Takashi Yokoo, and Clinical Associate Shuichiro Yamanaka of the Division of Nephrology and Hypertension, Department of Internal Medicine at the Jikei University School of Medicine has successfully applied artificial apoptosis induction technology to animal models. The research team selectively removed progenitor cells in early organ formation to develop kidney failure model mice in which the pathology of chronic kidney disease (CKD) is reproduced. This research is expected to contribute to elucidating CKD mechanisms and developing new treatments. Their findings were published in the international scientific journal Nature Communications on March 15.
Provided by the Jikei University
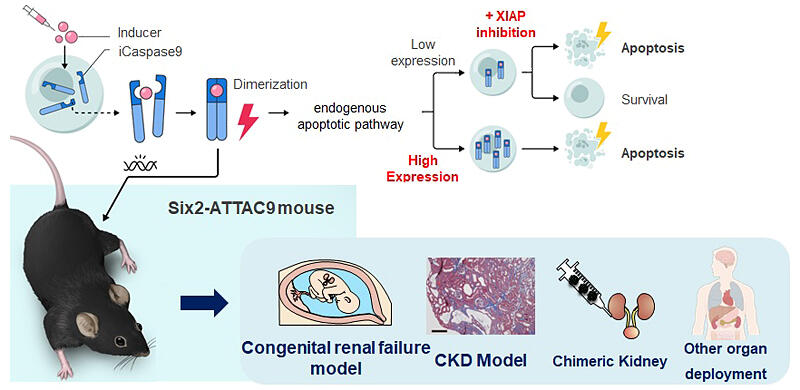
CKD is a disease that requires dialysis or kidney transplantation as it progresses, with an estimated 13.3 million patients in Japan. Until now, creating models of kidney failure that develop from the fetal stage has been technically difficult, posing a major challenge for research. In this study, the research group developed Six2-ATTAC9 mice by incorporating a system called "inducible Caspase9 (iCaspase9)" that artificially induces cell death.
Specifically, they focused on Caspase9, a major executor in the intrinsic apoptotic pathway, incorporating iCaspase9, which binds to artificially produced inducers. They successfully activated the apoptotic pathway according to inducer administration, selectively inducing apoptosis in Six2-positive nephron progenitor cells. Nephrons are functional structures that filter blood and produce urine, and these structures are destroyed in kidney disease. When the inducer is administered, iCaspase9 dimerizes and activates, eliminating Six2-positive nephron progenitor cells. Although high iCaspase9 expression is needed for this system to function, using a XIAP inhibitor, which has an apoptosis inhibitory effect, meant that the group was able to induce apoptosis even in cells with low iCaspase9 expression or in solid organ states with high apoptosis resistance.
Compared to conventional cell removal methods, this approach excels in speed and removal rate. By administering the inducer to pregnant mothers, they successfully reduced nephron progenitor cells in fetuses, inducing kidney failure.
By delaying the timing of inducer administration, the kidney failure becomes milder, allowing adjustment of kidney failure severity. Fetuses administered the inducer on gestational day 13.5 survived after birth, enabling detailed observation of chronic kidney dysfunction. These mice showed characteristics similar to human CKD patients, including glomerular enlargement and sclerosis, interstitial fibrosis, and inflammatory cell infiltration. This provides a useful model for elucidating CKD progression mechanisms. The group also successfully created chimeric kidneys incorporating human tissue by removing nephron progenitor cells from these fetal kidneys and injecting progenitor cells derived from human iPS cells.
Furthermore, they revealed for the first time in an animal model that solid organs show resistance to apoptosis compared to dissociated cell states.
Yamanaka commented: "Removing specific cells when organs are being formed according to the body's blueprint was extremely difficult. We succeeded in operating a special switch that was incorporated to make only targeted cells 'disappear.' This allows us to freely reduce the number of important kidney components (nephrons) and examine diseased kidneys in detail in the laboratory. We expect this technology to be useful for future kidney disease treatment and research to create new kidneys (such as regenerative medicine)."
Journal Information
Publication: Nature Communications
Title: Caspase 9-induced apoptosis enables efficient fetal cell ablation and disease modeling
DOI: 10.1038/s41467-025-57795-6
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




