A research group led by Associate Professor Wataru Shihoya from Keio University School of Medicine, The Sakaguchi Laboratory - Department of Signal Exploration (at the time of the research; now Assistant Professor at the University of Tokyo Graduate School of Science), Professor Osamu Nureki from the University of Tokyo Graduate School of Science, and Lassogen Inc. has successfully determined the binding structure of lasso peptide RES-701 to the endothelin type B (ETB) receptor using cryo-electron microscopy single-particle analysis. Their findings were published in Nature Communications.
Provided by the University of Tokyo, Keio University
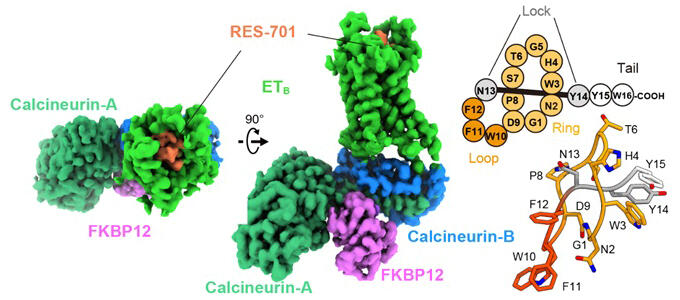
The ETB receptor, a G protein-coupled receptor (GPCR) found on cell surfaces, is known to regulate blood vessels and play roles in tumor angiogenesis and immune responses. This makes it a promising target for treating therapy-resistant cancers, with significant demand for therapeutic drug development. However, previous development efforts have struggled to produce small molecule compounds with sufficient regulatory activity and selectivity. While the lasso peptide RES-701 demonstrates higher selectivity for the ETB receptor than existing drugs and shows promise as an inverse agonist, understanding its mechanism of action on the ETB receptor has been a challenge for pharmaceutical applications.
In this study, the researchers applied a calcineurin fusion method to successfully determine the structure of the RES-701-bound ETB receptor, overcoming a challenge that was previously considered difficult. As a result, they visualized how the peptide binds to a specific hydrophobic pocket within the receptor. This binding inhibits the structural changes necessary for interaction between the G protein and the receptor, revealing the mechanism behind its inverse agonist activity.
This achievement opens new possibilities for developing medicines based on lasso peptides targeting the ETB receptor, with potential applications for treating various diseases, particularly immunotherapy-resistant cancers.
Journal Information
Publication: Nature Communications
Title: Structure of a lasso peptide bound ETB receptor provides insights into the mechanism of GPCR inverse agonism
DOI: 10.1038/s41467-025-57960-x
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




