A research group led by Professor Wataru Sakamoto from the Research Institute for Interdisciplinary Science at Okayama University, in collaboration with Professor Genji Kurisu and Associate Professor Akihiro Kawamoto from the University of Osaka's Institute for Protein Research, Senior Technical Scientist Kiminori Toyooka from the RIKEN Center for Sustainable Resource Science, and Professor Toru Terachi from Kyoto Sangyo University's Faculty of Life Sciences, has discovered how a protein called VIPP1 maintains thylakoid membranes where photosynthesis light conversion reactions occur. They successfully created plants with enhanced heat resistance using this protein. Their findings were published in Plant Physiology.
Gachie et al. The thylakoid membrane remodeling protein VIPP1 forms bundled oligomers in tobacco chloroplasts, Plant Physiology, Volume 198, Issue 1, May 2025, kiaf137, https://doi.org/10.1093/plphys/kiaf137. CC BY-NC-ND 4.0
Photosynthesis is the only biological process that can convert light energy into usable forms, producing oxygen and carbon compounds from water and carbon dioxide. This essential chemical reaction takes place in the sac-like thylakoid membranes within the chloroplast found only in photosynthetic organisms.
The VIPP1 protein, discovered 25 years ago, exists as a large complex in chloroplast thylakoid membranes and was predicted to be important for maintaining a photosynthetic apparatus that performs photosynthetic energy conversion and water-splitting. However, its detailed function remained unclear. Research in 2021 revealed that VIPP1 forms a structure composed of more than 100 bonded molecules. These findings indicated that VIPP1 is part of a universal protein family called ESCRT-III, which maintains various membrane functions through fusion and remodeling across all organisms. Plants produce VIPP1, a photosynthesis-specialized ESCRT-III protein, to maintain thylakoid membranes in response to environmental stresses such as strong light and high temperatures.
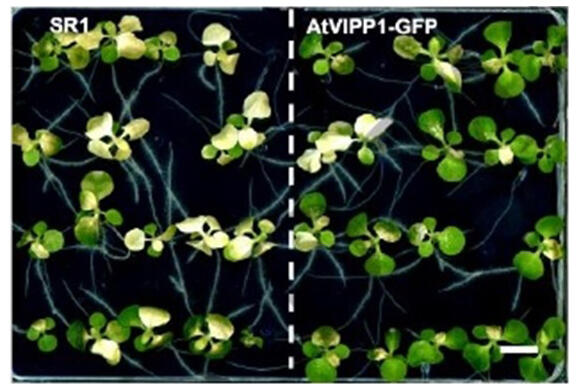
In the current study, the research group revealed the structure of VIPP1 accumulated in plant chloroplasts at a 0.1-micrometer level. First, they developed tobacco plants that accumulated 15 times more VIPP1 in chloroplasts than normal plants. The VIPP1 protein was tagged with GFP (green fluorescent protein).
Using electron tomography to analyze the GFP-visualized protein, they discovered that VIPP1 exists as bundles of elongated filamentous structures with diameters of 20-30 nanometers. When chloroplasts were exposed to high-temperature stress, these VIPP1 filamentous structures dynamically changed shape, released filaments and repeatedly underwent fusion and division between VIPP1 proteins, demonstrating membrane remodeling activity in response to membrane stress. When these tobacco plants were treated at 50℃, the VIPP1-overexpressing plants showed resistance, while wild-type plants suffered developmental defects.
Sakamoto commented: "Photosynthesis is an incredibly sophisticated reaction that uses light to create organic matter and energy from water and carbon dioxide, essential to our lives. I would like to understand the unknown photosynthetic capabilities of plants and design photosynthesis. By the way, leaves appear green because of thylakoid membranes, which produce the oxygen on Earth."
Journal Information
Publication: Plant Physiology
Title: The thylakoid membrane remodeling protein VIPP1 forms bundled oligomers in tobacco chloroplasts
DOI: 10.1093/plphys/kiaf137
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




