Associate Professor Takato Mitsudome and third-year Doctoral Student (at the time of the research) Tomohiro Tsuda from the Graduate School of Engineering Science at the University of Osaka and their colleagues have discovered that iron phosphide nanocrystals (Fe2P NC), made of iron and phosphorus, can function as highly efficient catalysts for the reductive amination of carbonyl compounds, an important process in amine synthesis. When supported on zirconia, this iron phosphide nanocrystal catalyst showed more than 300 times higher activity than conventional iron catalysts. The reaction efficiently produces target primary amines from various carbonyl compounds with high yields, and the catalyst maintains its high activity for reuse after the reaction. The group's findings were published in the Journal of the American Chemical Society.
Provided by the University of Osaka
The reductive amination of carbonyl compounds using ammonia as a nitrogen source is an industrially important reaction for synthesizing primary amines, which are essential as pharmaceutical intermediates and polymer materials. Catalysts that promote this reaction typically use rare and expensive precious metals or metals like nickel and cobalt whose depletion is a concern, creating demand for catalyst development using iron, which is more inexpensive and versatile.
The research group has been working on synthesizing various metal nano compounds through their original methods and has now successfully synthesized rod-shaped iron phosphide nanocrystals (Fe2P NC) composed of iron and phosphorus.
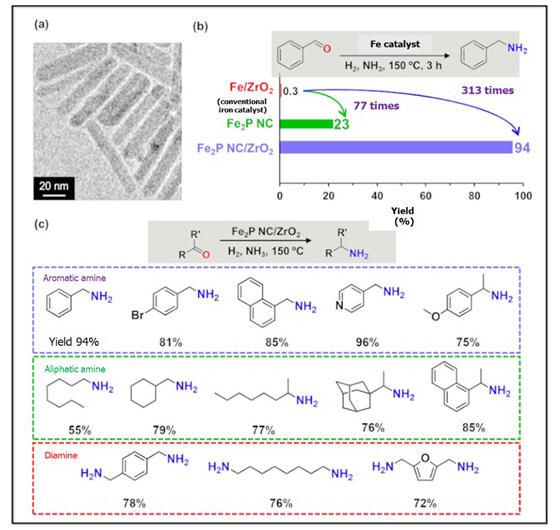
These iron phosphide nanocrystals function as catalysts that promote the reductive amination of aldehydes, showing 77 times higher catalytic activity compared to conventional iron catalysts (Fe/ZrO2). Additionally, unlike conventional iron nanoparticles, the rod-shaped iron phosphide nanocrystals are stable in air, and when combined with ZrO2, their catalytic activity was dramatically improved, achieving extremely high activity—313 times that of conventional iron catalysts.
This composite catalyst can obtain amines with high yields in reductive amination reactions of various carbonyl compounds, and these amines can be utilized as pharmaceutical intermediates and polymer materials. Furthermore, since this catalyst is solid, it can be easily recovered by centrifugation after the reaction and maintains high activity even after reuse.
This marks the world's first successful development of an iron-based catalyst for reductive amination reactions that combines high activity and durability in reductive amination reactions. The paper also details the elucidation of factors contributing to the composite catalyst's activity and the reaction mechanism.
Mitsudome commented: "To realize a sustainable society, we need to revise material selection and foundational technologies with resource circulation in mind. In particular, utilizing 'iron,' which is abundant and has a low environmental impact, as a catalyst has long been considered ideal, but practical solutions have not been found for iron's instability and low activity. In this research, we demonstrated one direction for developing practical iron catalysts to address these challenges."
Journal Information
Publication: Journal of the American Chemical Society
Title: Highly Active and Air-Stable Iron Phosphide Catalyst for Reductive Amination of Carbonyl Compounds Enabled by Metal-Support Synergy
DOI: 10.1021/jacs.4c18611
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




