The research group of Assistant Professor Mitsuaki Yamauchi, Doctoral Student So Ueno, Assistant Professor Keitaro Yamamoto, Associate Professor Yoshiyuki Mizuhata, and Professor Hiroko Yamada from the Institute for Chemical Research at Kyoto University, in collaboration with Assistant Professor Nobutaka Shioya, Senior Researcher Hiroshi Matsuda, and Professor Takeshi Hasegawa, successfully developed an organic thin-film transistor with a hydrogen bond network through a solution-coating process. While hydrogen bonds enable clear bond orientation and precise supramolecular structural control compared to van der Waals forces, their introduction typically reduces solubility for solvents, limiting transistor applications. By employing a thermal precursor approach using a highly soluble precursor, the group developed the first transistor composed of hard-to-dissolve hydrogen-bonded tetrabenzoporphyrin. In addition to charge mobility comparable to amorphous silicon, they demonstrated excellent thermal durability through hydrogen bonding and elucidated the molecular orientation and 2D packing structure within the thin film. The research provides a pathway for structural analysis of supramolecular thin films, offers design guidelines for previously underdeveloped hydrogen-bonded transistors, and is expected to promote supramolecular device development. It was published in Angewandte Chemie International Edition.
Provided by Kyoto University
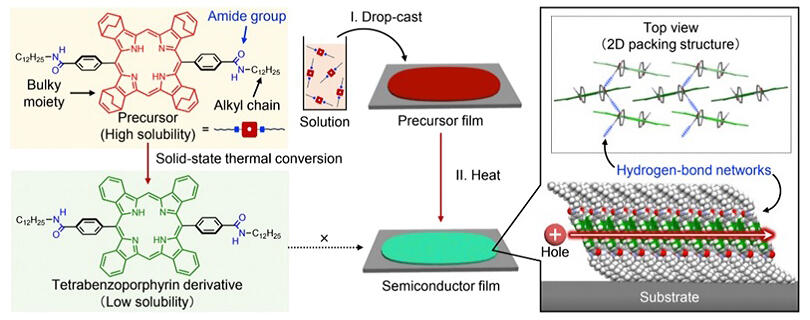
By incorporating a thin-film fabrication approach using a highly soluble thermal precursor (thermal precursor approach), the research group successfully developed an application of a difficult-to-dissolve compound - compound in which amide groups and alkyl chains are incorporated into rigidπ-extended tetrabenzoporphyrin (BP) - as an organic thin-film transistor.
Specifically, they synthesized a soluble precursor of BP with bulky substituents, drop-casting its chloroform solution onto a substrate and drying it to readily create a precursor thin film. By heating this precursor film, they converted it into a polycrystalline BP film and deposited gold electrodes to fabricate transistor devices. Although polycrystalline films, unlike single crystals, typically have multiple crystal boundaries that could potentially hinder charge transport, resulting in significant charge mobility reduction, the devices actually demonstrated hole mobility comparable to amorphous silicon. This is believed to be because the hydrogen bond network plays a "glue-like" role at crystal boundaries, contributing to maintaining continuous charge transport pathways.
Furthermore, by virtue of this hydrogen bond network, the transistor devices demonstrated high thermal durability even after heating in air at 250℃, ensuring sufficient device performance. Using X-ray structural analysis and multiple-angle incidence resolved spectroscopy, the group detailed the molecular orientation and intermolecular interactions in the BP film. It was revealed that BP molecules form a "twisted stacking" structure and aggregate in a two-dimensional direction through hydrogen bonding. This unique assembly structure is considered to have contributed to the relatively high hole mobility.
By utilizing the thermal precursor approach to indirectly form hard-to-produce insoluble organic thin films, the group were able to develop research on previously underdeveloped hydrogen-bonded transistors. This method is expected to be applicable to other insoluble compounds and contribute to further development and activation of the supramolecular electronic device field.
It is also considered usable as a future roadmap for structural analysis of supramolecular thin films. Going forward, the group plans to explore strategies to maximize charge mobility while developing functionality responsive to external stimuli such as light and temperature, aiming to maximize the potential of hydrogen-bonded organic semiconductor thin films.
Yamauchi commented, "Aiming to meaningfully merge supramolecular chemistry, my specialized field, with organic semiconductors, I conceived this research theme about two years ago and have been advancing it with the first author, Mr. Ueno. To achieve the research objectives, there were many challenges to solve, including solubility issues, barriers to device fabrication, and difficulties in structural analysis. Fortunately, by closely collaborating with researchers inside and outside the laboratory and concentrating and interacting with expertise from various fields, we were able to realize our long-desired hydrogen-bonded transistor."
Journal Information
Publication: Angewandte Chemie International Edition
Title: Hydrogen-Bond-Directed Supramolecular Organic Semiconductor Thin Films Realized via Thermal Precursor Approach
DOI: 10.1002/anie.202425188
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




