Ammonia has been widely used as a raw material supporting diverse industrial activities, including fertilizers and chemical products. In recent years its use as an energy carrier and as fuel that can be stored and transported at higher energy density than hydrogen gas has been studied worldwide, due to its properties of not emitting carbon dioxide during combustion and being easily liquefied with slight pressurization or cooling.
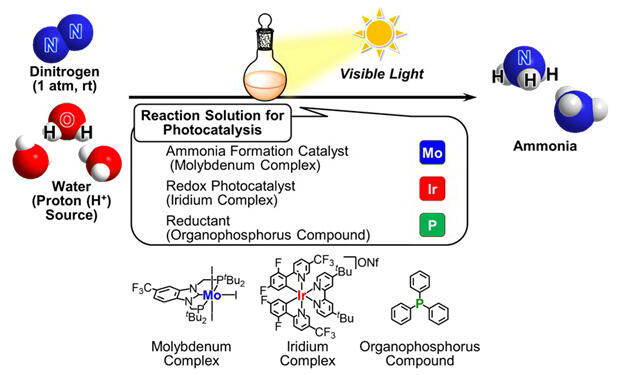
A research group led by Professor Yoshiaki Nishibayashi from the Graduate School of Engineering at the University of Tokyo has successfully achieved photocatalytic synthesis of ammonia (NH3) from nitrogen gas (N2) and water (H2O) under ambient temperature and pressure conditions using visible light irradiation, the main component of sunlight. They used two types of molecular catalysts: molybdenum complexes as ammonia synthesis catalysts and iridium complexes as photoinduced electron transfer catalysts, along with organophosphorus compounds such as triphenylphosphine (Ph3P) as reducing agents. Their research was published in Nature Communications.
Provided by the University of Tokyo
In 2018, the research group discovered that when photoreactions induce electron transfer from organophosphorus compounds to other molecules (photoinduced electron transfer), P-O bonds are formed with water molecules. They revealed that this bond formation activates the water molecules, enabling them to function as strong acids that can easily donate protons to other molecules.
In this study, applying insights gained from this reaction system, they aimed to use water as a proton source for ammonia synthesis reactions proceeding on molybdenum complexes by activating water molecules through photoinduced electron transfer from organophosphorus compounds using photoinduced electron transfer catalysts with high photooxidation power.
Specifically, when they irradiated visible light on a solution containing molybdenum complexes as ammonia synthesis catalysts, iridium complexes as photoinduced electron transfer catalysts, organophosphorus compounds as reducing agents, and water as a proton source under a nitrogen gas atmosphere at room temperature and atmospheric pressure, ammonia was catalytically produced. In this reaction system, it was revealed that adding a proton mediator (pyridine derivative) that can promote proton transfer between substrates and catalysts dramatically improved the reaction activity.
The group achieved the highest catalytic turnover number among photocatalytic ammonia synthesis reaction systems reported to date, while successfully improving the quantum efficiency dramatically from 2% in previous studies to 22%.
Investigation of the reaction mechanism of this photocatalytic reaction suggested that it proceeds through multiple processes. First, using the energy of irradiated visible light, the iridium complex serving as a photoinduced electron transfer catalyst induces electron transfer from the organophosphorus compounds, resulting in the iridium complex becoming reduced and the organophosphorus compounds becoming oxidized. The oxidized organophosphorus compounds form bonds with water molecules, activating the water molecules.
Meanwhile, the molybdenum complex activates nitrogen molecules to generate nitride complexes with nitride ligands. Ammonia is produced when the reduced iridium complex donates electrons and the organophosphorus compounds bonded to water molecules donate protons to the nitride complex (proton-coupled electron transfer). During this process, proton transfer from organophosphorus compounds bonded to water molecules to the nitride complex is significantly accelerated in the presence of proton mediators.
This research represents the world's first successful example of synthesizing ammonia from nitrogen gas and water at room temperature and atmospheric pressure using visible light, which is the main component of sunlight. The demand for ammonia is expected to expand toward achieving carbon neutrality. This work provides important insights for developing green ammonia synthesis methods and is expected to have significant ripple effects across various research fields.
Journal Information
Publication: Nature Communications
Title: Catalytic ammonia formation from dinitrogen, water, and visible light energy
DOI: 10.1038/s41467-025-59727-w
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




