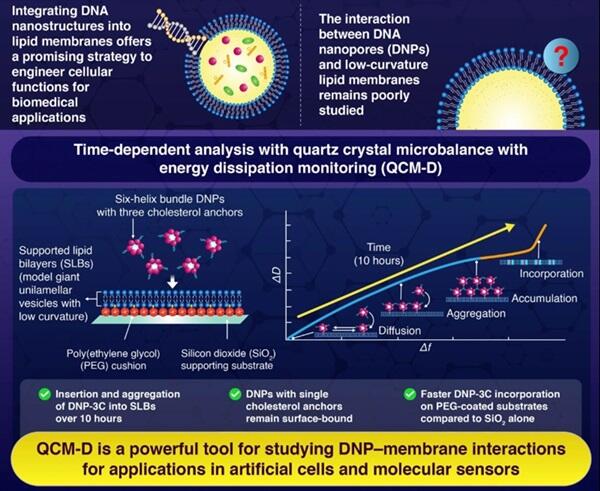
A research team led by Associate Professor Tomohiro Hayashi, Doctoral Student Zugui Peng, and Professor Tohru Yagi from the Department of Materials Science and Engineering, School of Materials and Chemical Technology at the Institute of Science Tokyo has achieved the world's first high-temporal-resolution, non-invasive observation of interactions between DNA nanopores (DNPs) and lipid bilayers, as well as the time-dependent changes in membrane mechanical properties caused by these interactions, using QCM-D (quartz crystal microbalance with dissipation). As a result, the team continuously tracked how cholesterol-modified DNA nanopores are adsorbed and incorporated into lipid membranes over several hours, discovering that differences in supporting substrates (silicon oxide films and polyethylene glycol) significantly influence these dynamics. Furthermore, they clarified DNA aggregation behavior during the incorporation process and changes in membrane viscoelasticity, paving the way for applications in artificial cells and molecular sensors. Their research was published in Nanoscale.
Unraveling the time course of interaction between DNA nanopores and lipid bilayers using QCM-D: role of cholesterol anchors and bilayer supporting substrates, Peng et al. (2025),
Nanoscale, DOI: 10.1039/d5nr01299f
DNA nanopores are attracting attention as artificial channels that enable the transport of drugs, ions, and other substances through biological membranes such as cell membranes, making substance transportation possible within the body. However, since DNA is hydrophilic and negatively charged, an energy barrier exists between DNA and lipid bilayers, which similarly have hydrophilic and negatively charged surfaces, making it difficult to incorporate DNA nanopores into membranes.
The research group designed DNA nanopores that differ from conventional nanopores by having hydrophobic cholesterol groups (tags) around the molecules. They also constructed a system that changed the membrane support environment by introducing polymer films between the lipid bilayers and the supporting substrate (silicon oxide). Using these systems, they carried out QCM-D measurements and captured in real-time the process of DNA nanopore adsorption and incorporation into lipid membranes as nanogram-level mass changes and viscoelastic changes.
In particular, DNA nanopores with three hydrophobic cholesterol groups attached to their molecular surfaces showed higher adsorption than DNA nanopores with only one cholesterol group and made the entire membrane stiffer. Additionally, it was revealed that coating the silicon oxide supporting substrate with the polymer polyethylene glycol (PEG) significantly increased the incorporation speed of DNA nanopores.
This revealed that by combining controls of the environment in which nanopores and lipid bilayers are placed, it is possible to control DNA nanopore incorporation and changes in membrane mechanical properties.
These results will provide design guidelines for efficiently incorporating DNA structures into membranes by changing the quantities and interactions of DNA structures in the development of biodevices such as artificial cells and molecular sensors that perform substance transport through lipid bilayers.
In the future, the team plans to conduct similar evaluations for DNA structures with different shapes and modifications, aiming to generalize these design guidelines. Intracellular substance delivery and membrane sensor development are expected as applications of membrane-penetrating nanopores, and research will proceed with synthetic biology and diagnostic medicine applications in mind.
Journal Information
Publication: Nanoscale
Title: Unraveling the time course of interaction between DNA nanopores and lipid bilayers using QCM-D: role of cholesterol anchors and bilayer supporting substrates
DOI: 10.1039/d5nr01299f
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




