A research group led by Assistant Professor Tatsuhiko Yokoyama, Associate Professor Tomoko Kubori, and Professor Hiroki Nagai from the Graduate School of Medicine at Gifu University, along with Professor Yoshinori Akiyama from the Institute for Life and Medical Sciences at Kyoto University, in collaboration with Nara Institute of Science and Technology and RIKEN, has revealed that bacteria not only use the mechanical force generated by molecular motors to transport iron into cells but also utilize this force to sense iron. Their findings were published in the online version of PNAS.
Provided by Gifu University
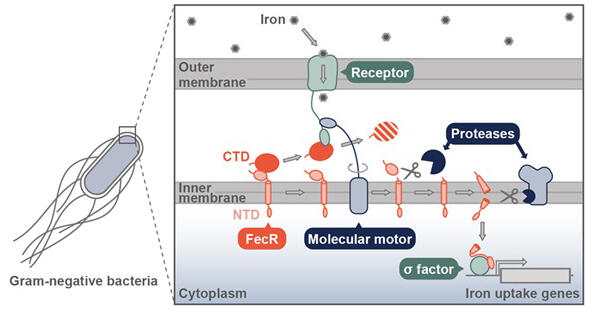
Gram-negative bacteria are separated from the external environment by outer and inner membranes. Molecular motors exist in their inner membranes and generate mechanical force by utilizing the proton motive force. This mechanical force enables receptor proteins located in the outer membrane to capture external iron and draw it into the cell. While research on the mechanisms of iron uptake into cells has progressed, how bacteria sense iron present in the external environment, transmit this information to the cell interior, and induce the expression of genes necessary for iron uptake remained unknown.
The research group discovered that the mechanical force generated by molecular motors is also transmitted to the membrane protein FecR located in the inner membrane. Specifically, they first found that FecR is inserted into the inner membrane in a state separated into two regions: the NTD region and the CTD region. The mechanical force from the molecular motor is transmitted to the CTD region of FecR via receptor proteins, causing the CTD region to dissociate from the NTD region. Once the CTD region dissociates, the NTD region undergoes two consecutive cleavages by proteases. Finally, a small fragment generated from the NTD translocates from the membrane to the cell interior and activates the sigma factor, inducing the expression of genes that constitute the iron uptake system and enabling bacteria to more efficiently take up iron from the external environment.
In this study, the researchers elucidated the molecular mechanism by which information from outside the cell is transmitted into the cell through the precisely controlled sequential cleavage of membrane proteins. Signal transduction via protein cleavage is observed not only in bacteria but also in many other species including humans and is involved in important biological phenomena such as cell death, cholesterol synthesis, and the onset of neurodegenerative diseases. This research has revealed a new mechanism of signal transduction involving protein cleavage, shedding light on one aspect of the fundamental mechanisms controlling biological functions.
Yokoyama commented: "The existence of the signal transduction system studied in this research has been known for nearly half a century. However, the molecular mechanism by which FecR, the core of this system, transmits information remained a mystery. In this study, we unraveled the process by which FecR is synthesized in the cell, inserted into the membrane, and finally degraded — in other words, the 'life' of the FecR protein — through detailed biochemical analysis, and clarified the entire signal transduction mechanism. I believe that this research will serve as an essential foundation for advancing the study of similar types of signal transduction mechanisms in the future. Finally, I would like to express my sincere respect for Dr. Volkmar Braun of the Max Planck Institute for Biology, who has promoted numerous studies that formed the foundation of this research and made significant contributions to the development of this research field."
Journal Information
Publication: PNAS
Title: Cleavage cascade of the sigma regulator FecR orchestrates TonB-dependent signal transduction
DOI: 10.1073/pnas.2500366122
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




