A research group led by Professor Yasushi Kawaguchi, Associate Professor Akihisa Kato, and Graduate Student Hayato Harima (at the time of the research, currently Assistant Professor at the Institute of Agriculture, Tokyo University of Agriculture and Technology) from the Department of Microbiology and Immunology at the Institute of Medical Science, the University of Tokyo has revealed that the viral uracil-DNA glycosylase (vUNG) encoded by herpes simplex virus 1 (HSV-1) promotes the development of herpes encephalitis by counteracting the function of the host's intrinsic immune effector APOBEC1 in the central nervous system. Furthermore, based on this finding, they proposed the possibility of a new treatment strategy that could revive the host's intrinsic immunity using a viral vector targeting vUNG, leading to the suppression of lethal encephalitis. Their findings were published in Nature Microbiology.
Provided by the University of Tokyo
Herpesviruses have established a highly sophisticated symbiotic relationship with their hosts through over 400 million years of co-evolution. However, herpes encephalitis caused by HSV-1 is an exceptionally lethal disease. This suggests that HSV-1 cleverly evades the host's immune response in the central nervous system. Therefore, elucidating the interaction between the host's immune response in the brain and HSV-1's evasion mechanism is extremely important for understanding the pathogenesis of herpes encephalitis and developing new therapeutic approaches.
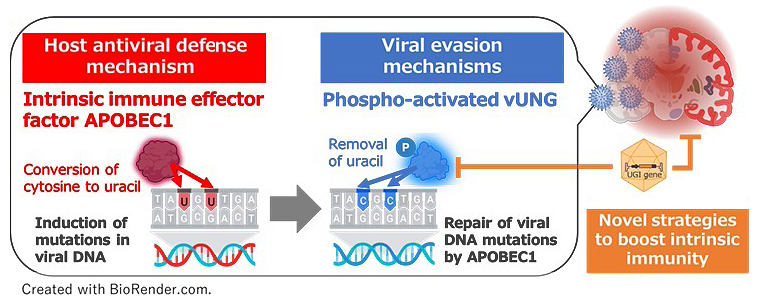
The research group first revealed through phosphoproteomic analysis that Ser-302 of vUNG is phosphorylated. When they created a vUNG mutant virus (vUNG-S302A) in which this phosphorylation site was replaced with alanine, the enzymatic activity of vUNG was almost completely lost in infected cells, and both viral production in mouse brains and mouse lethality were significantly reduced. This result indicates that phosphorylation of vUNG at Ser-302 is important for both enzymatic activity and the development of herpes encephalitis.
Furthermore, the induction of APOBEC1 expression in mouse brains by HSV-1 infection suggested that APOBEC1 might convert cytosine to uracil within the viral genome, introducing mutations. Indeed, APOBEC1-dependent DNA mutations were detected on the viral genome in wild-type mice infected with the vUNG-S302A mutant virus. In contrast, no DNA mutations were detected in APOBEC1-deficient mice or in infections with viruses containing phosphorylated vUNG, and the reduction in lethality by the vUNG-S302A mutant virus was not observed. This revealed that vUNG suppresses the antiviral activity of APOBEC1 in the central nervous system and contributes to efficient development of herpes encephalitis.
Finally, as the small molecule UGI derived from bacteriophage is known to inhibit vUNG, they created an adeno-associated virus vector expressing UGI (AAV-UGI). When this AAV-UGI was administered to wild-type mice followed by HSV-1 infection, mouse lethality was significantly reduced. In contrast, no lethal suppression effect by AAV-UGI was observed in APOBEC1-deficient mice. This result indicates that by inhibiting vUNG, intrinsic immunity mediated by APOBEC1 can be restored, potentially suppressing herpes encephalitis.
This study demonstrated the possibility of reviving the host's "inherent viral resistance" and connecting it to the treatment of viral diseases by targeting the immune evasion mechanism (vUNG) that viruses have acquired through evolution. In particular, the in vivo analysis of the interaction between the viral factor vUNG and the host factor APOBEC1, and the elucidation of their functions through modifications of both viral and host genomes, represents an extremely important academic achievement. Furthermore, the demonstration that intrinsic immunity can be a new antiviral therapeutic target provides an important pathway toward creating next-generation antiviral strategies.
Journal Information
Publication: Nature Microbiology
Title: Herpes simplex virus 1 evades APOBEC1-mediated immunity via its uracil-DNA glycosylase in mice
DOI: 10.1038/s41564-025-02026-3
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




