A research group led by Senior Program Advisor (Professor Emeritus) Hajime Tanaka and Project Researcher Gang Sun (at the time of the research) from the Frost Protection Science Social Cooperation Program at the Institute of Industrial Science, the University of Tokyo has published research findings that provide a new understanding of the long-standing mystery of how ice forms on solid surfaces.
Ice formation (ice nucleation) is an extremely important phenomenon in the fields of atmospheric science, biophysics, and materials science. It is involved in a wide range of phenomena from cloud formation and aircraft icing to freeze preservation and protein crystallization. Most ice nucleation in nature occurs in environments where surfaces are present; this is known as heterogeneous nucleation, and elucidating its microscopic mechanisms holds the key to controlling ice formation.
The conventional theoretical framework, Classical Nucleation Theory (CNT), has described the probability of ice nucleus formation using macroscopic thermodynamic quantities such as interfacial free energy and wetting angles (contact angles). However, this theory cannot adequately capture the molecular-scale interactions between liquids and solids or the ordered nature of liquid structures. Moreover, actual nucleation behavior sometimes deviates significantly from CNT predictions.
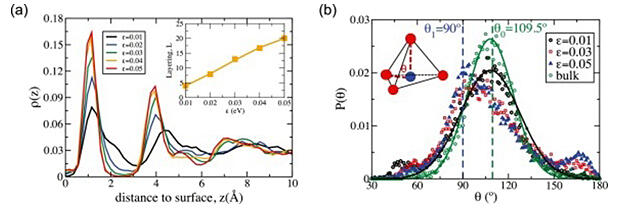
(a) Layered structure of liquid water near the solid surface. Density profiles of liquid water at T = 210 K before ice nucleation, for different interaction (hydrophilicity) strengths ε = 0.01, 0.02, 0.03, 0.04, and 0.05 eV. Inset: The degree of layering L of liquid water adjacent to the substrate increases with increasing ε.
(b) Distribution of the angle θ formed by a water molecule in the contact layer and two neighboring molecules connected by dashed lines (see inset). The green and blue dashed lines indicate the positions of the primary peaks at θ0 = 109.5° and θ1 = 90°, respectively.
Provided by the University of Tokyo
The research group carried out molecular dynamics simulations using water models that can accurately reproduce the behavior of liquid water at the atomic scale, tracking the initial processes of ice formation in detail. They employed a simple cubic lattice for the solid substrate and controlled the interaction strength (hydrophilicity) with atoms to comprehensively investigate a wide range of surface properties. They particularly focused on the formation process of "liquid ordered structures," which are considered precursor states to ice.
The analysis revealed that changes in water structure near solid surfaces play an important role in surface-induced crystallization. As the attractive interaction between water near the surface and the solid becomes stronger, the water is drawn to the surface, resulting in a layered structure. Furthermore, the hydrogen bond angles were found to change significantly from the natural 109.5 degrees to 90 degrees. Reflecting these changes in the liquid structure of water, it became clear that ice nucleus formation is not a simple one-step process but rather a hierarchical structuring process.
For example, in the case of intermediate hydrophilicity where ice forms most easily, molecules first align so they are flat in the contact layer (first layer) in contact with the surface, and a network structure centered on six-membered rings appears. Subsequently, similar structures are induced in the upper layer (second layer), and eventually ice crystals grow across regions spanning three or more layers, following a scenario of "two-dimensional preordering → three-dimensional crystal growth." When hydrophilicity is too high, water molecules excessively adsorb to the surface, suppressing the formation of ordered structures. Conversely, when hydrophilicity is too low, contact is insufficient, and structuring does not progress. These results demonstrate that, in addition to lattice matching between ice and substrate crystals, the "local structural order of liquid water" drives nucleation, emphasizing the importance of a new perspective (crystal precursor-induced crystallization mechanism) that complements conventional classical nucleation theory.
Furthermore, it was revealed that the liquid structures that form near surfaces are low-dimensional (quasi-two-dimensional) ordered structures, different from so-called three-dimensional ice structures. This order forms even on solid surfaces that do not have direct structural compatibility with ice, and its presence or absence strongly influences ice nucleation. This presents a fundamentally different paradigm from conventional discussions based on "similarity between ice and substrate crystals."
These insights are likely also applicable to liquids with tetrahedral structures such as silica and silicon, leading to the construction of a universal understanding of surface-induced crystallization. Furthermore, in machine learning-based surface design, which has recently attracted attention, molecular-scale mechanical and structural insights complement interpretability and versatility.
In the future, starting from the insights of hierarchical structuring of liquids near surfaces, there are expectations of analysis of nucleation behavior on more complex real surfaces (such as atmospheric mineral particles and biomolecule surfaces) and development of controlled ice generation and suppression technologies.
Tanaka commented: "While ice is a familiar presence, understanding the moment of its birth at the molecular level has long been difficult. This research has revealed that the essence of this is not that 'ice-like surfaces' are important, but rather how surfaces nurture 'water that wants to become ice.' Moving forward, we would like to leverage these insights to connect to technologies that can freely control 'the birth of ice' across a wide range of fields from climate models to freeze preservation."
Journal Information
Publication: Journal of Colloid and Interface Science
Title: The secret role of water's structure near surfaces in ice formation
DOI: 10.1016/j.jcis.2025.137812
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




