A research group led by Professor Shigeki Kiyonaka and Graduate Student Kyohei Soga from the Graduate School of Engineering at Nagoya University, in collaboration with Professor Eriko Nango from the Institute of Multidisciplinary Research for Advanced Materials at Tohoku University, announced the development of a molecule called "PFQX1(AF488)" for the visualization of AMPA-type glutamate receptors in synapses. This probe can be used to rapidly label these receptors in approximately 10 seconds simply by adding it to a culture medium. The labeling can be easily removed without affecting receptor function, and re-labeling is possible. Using this molecule, the research team successfully conducted dynamic analysis of these receptors. The results are expected to contribute to analysis of memory mechanisms and developing treatments for diseases. The group's findings were published in the international academic journal Science Advances on June 7.
Provided by Nagoya University
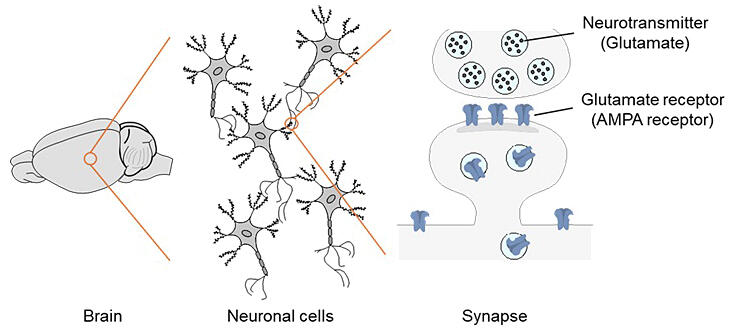
The brain serves as the center for learning and memory and consists of approximately 100 billion nerve cells. Nerve cells are connected to each other through synapses, and information transmission between synapses occurs through the transfer of neurotransmitters to receptors. Each nerve cell has hundreds of thousands of synapses.
Memory is believed to be stored in synapses, and when learning occurs, a phenomenon called long-term potentiation (LTP) takes place in synapses, creating stronger connections. During LTP, glutamate is released from presynaptic cells and received by AMPA-type glutamate receptors (AMPA receptors) on postsynaptic cell membranes. During LTP, the number of these receptors increases, and the receptors show behavior where they move in and out of synapses. However, how this behavior relates to memory and learning remains unclear.
Two types of movement by these receptors have been reported: lateral diffusion, where receptors move across the membrane from dendrites away from synapses, and exocytosis, where receptors are released from the synapses themselves. The debate over which is the main type of movement has continued.
While genetic engineering methods exist to introduce fluorescent proteins (GFP) into receptors, concerns have been raised that GFP, being about one-quarter the size of AMPA receptors, might interfere with their movement.
The research group therefore aimed to develop probes specifically for observing AMPA receptors. They designed two types of probes that link ligands capable of binding to AMPA receptors with fluorescent molecules for visualization: one with a long distance from ligand to fluorescent molecule, and another with direct, short linkage. Each was added to human-derived cell lines that forcibly expressed AMPA receptors and observed using confocal laser microscopy.
Results showed that the short probe "PFQX1(AF488)," which directly connects the fluorescent molecule to the ligand, had higher binding affinity. Subsequent X-ray crystal structure analysis revealed the structure the complex of "PFQX1(AF488)" and AMPA receptors and showed that the fluorescent molecule itself interacts with the protein to further improve affinity.
To verify the function of the probe developed in this study, the group extracted and cultured mouse embryonic hippocampus and added the probe. Receptors were detected in approximately 10 seconds. Furthermore, they found that washing with buffer solution removed the probe from receptors, and re-addition enabled visualization again with the same fluorescent intensity.
Using the probe obtained, they artificially induced LTP and observed the results, confirming that this procedure led to AMPA receptor accumulation in synapses. They also verified that this accumulation was suppressed by LTP inhibitors.
To further elucidate LTP mechanisms, they investigated the visualization of AMPA receptor dynamics. The research group combined the two-step labeling method, which they developed in 2021 for labeling cell membrane surface receptors, with "PFQX1(AF488)" visualization to investigate changes after LTP induction. The results revealed that LTP occurs primarily through exocytosis.
Journal Information
Publication: Science Advances
Title: Rapid and reversible fluorescent probe enables repeated snapshot imaging of AMPA receptors during synaptic plasticity
DOI: 10.1126/sciadv.adt6683
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




