A research team led by Junior Associate Professor Akihito Inoko from the Department of Pathology, Aichi Medical University announced the development of a new method for long-term in vitro proliferation of immature basal cells that form the basis of human epithelial tissue and their maturation (differentiation) in a short period. By comparing the cells before and after differentiation using this method, the team found commonalities between immature normal basal cells and cancer. The method is expected to be applied to various medical developments including regenerative medicine and cancer research. Their results were published in the international academic journal Scientific Reports on April 9, 2025.
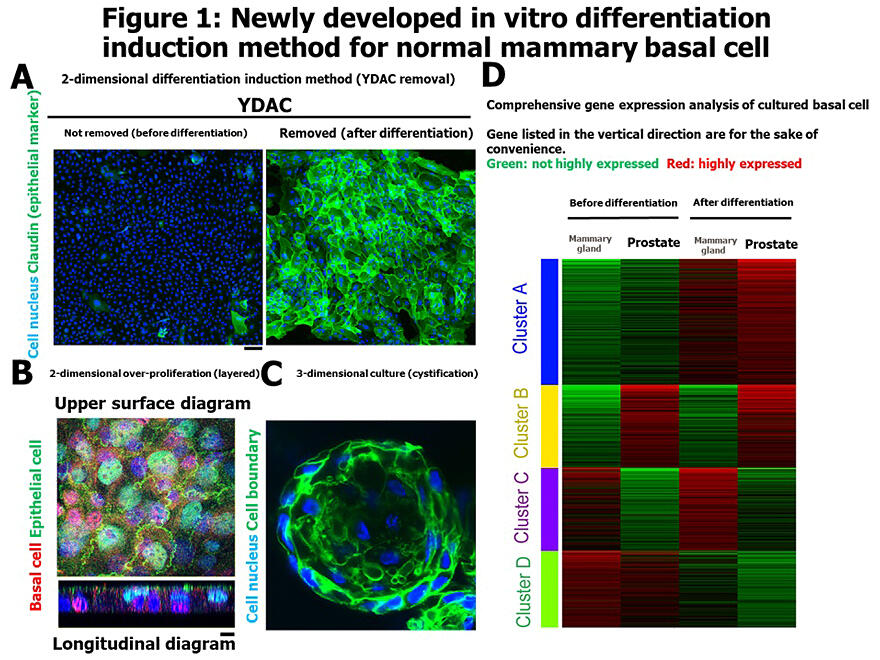
A: Epithelial differentiation while remaining two-dimensional through YDAC removal.
B: Layered structure similar to biological tissue.
C: Can also differentiate into three-dimensional cystic structures.
D: Gene expression analysis before and after differentiation.
Inoko A. et al. Scientific Reports (2025). CC BY 4.0
Epithelial tissue is an important barrier that protects the body from the external environment, and this barrier function is maintained by cell-to-cell junction structures called "tight junctions" specific to differentiated epithelium. The major protein "Claudin" was first reported by Kyoto University in 1998 and was known to be essential for epithelial barrier formation. However, it was difficult to differentiate immature epithelium in vitro in a way that could be easily reproduced, and the behavior during the maturation process remained unclear.
Therefore, the research group developed a new culture medium by adding a cocktail of four drugs named YDAC to F medium, which is commonly used for primary culture. With this medium, they successfully achieved long-term proliferation of human mammary basal cells in vitro. Furthermore, they revealed that when the medium was switched to one without the compounds, the cells could efficiently differentiate into epithelium in a short period while remaining in a flat two-dimensional state. YDAC is a mixture of ROCK inhibitor Y-27632, SMAD inhibitors DMH1 and A-83-01, and Wnt activator CHIR99021.
The group also confirmed that when basal cells were over-proliferated in two dimensions, they formed layered structures similar to biological tissues. They also confirmed that when cultured using existing organoid methods, the cells could differentiate into three-dimensional cystic structures closer to living organisms.
Gene expression analysis before and after differentiation showed increased expression of gene groups characteristic of mammary epithelium after differentiation. The group found that the transcription factor EGR1 was strongly expressed before differentiation, while ELF3 was strongly expressed after differentiation.
Experiments removing each of these factors showed that removing EGR1 from immature basal cells caused Claudin expression, while removing ELF3 from mature epithelial cells suppressed Claudin localization. It became clear that each transcription factor is involved in Claudin expression suppression and localization.
EGR1 expression and ELF3 deletion are known to be related to epithelial-mesenchymal transition (EMT: involved in invasion and metastasis) in cancer, revealing connections between cancer and normal basal cells. This result was also confirmed in mammary tissue and organoid methods.
Inoko commented: "Since encountering cell biology in medical school, I have always wanted to create a cellular analysis system close to clinical practice. The advances in iPS and organoids also provided encouragement, and this time we have developed a simple and clear modality with analytical power. Currently, we are working on applications to cancer culture and sensing a good response. If you are interested in collaborative research, please visit ResearchMap (https://researchmap.jp/inoko?lang=en)."
Journal Information
Publication: Scientific Reports
Title: Long-term expansion of basal cells and the novel differentiation methods identify mechanisms for switching Claudin expression in normal epithelia
DOI: 10.1038/s41598-025-95463-3
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




