A research group consisting of Senior Researcher Yui Fujihara, Senior Researcher Dai Kutsuzawa, and Principal Researcher Takeshi Kobayashi from the Energy Transformation Research Laboratory at the Central Research Institute of Electric Power Industry (CRIEPI) has developed a multilayer special cell that introduces additional electrodes serving as both reference electrodes (electrodes used as a reference when measuring electrode potential) and charge state adjustment electrodes in all-oxide solid-state batteries. On May 30 the research group announced that they successfully clarified the degradation factors of all-oxide solid-state batteries. Their results were published in ACS Applied Energy Materials issued by the American Chemical Society on April 14.
Provided by the Central Research Institute of Electric Power Industry
In recent years, all-oxide solid-state batteries using solid oxides as electrolytes and electrode active materials for both positive and negative electrodes have been anticipated as the next generation of power storage. However, researchers faced the challenge of being unable to perform detailed evaluations of the electrochemical properties of solid-state batteries due to their robust structure. Common practices in conventional liquid lithium-ion batteries, such as introducing reference electrodes and extracting electrodes from batteries in specific charge states, were difficult to achieve.
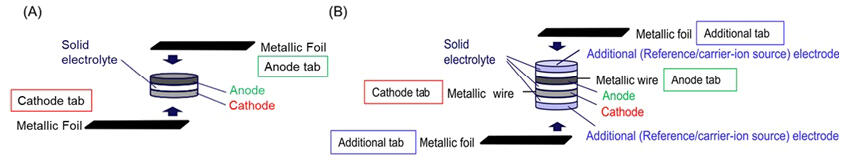
To address this challenge, the research group devised a multilayer special cell that adds two sets of electrolyte and electrode combinations to the conventional all-oxide solid-state battery consisting of three layers: positive electrode, electrolyte, and negative electrode. This enables detailed electrochemical evaluation similar to that of liquid lithium-ion batteries in all-oxide solid-state batteries.
There is a type of sodium-ion battery with an Na3Zr2(SiO4)2(PO4) solid electrolyte layer (a NASICON (Na Super Ionic CONductor) type oxide) and Na3Ti2(PO4)3 electrode active material (also a NASICON type oxide) for both positive and negative electrodes. For this type of battery, they fabricated a multilayer special cell by adding two sets of additional electrodes consisting of Na3Zr2(SiO4)2(PO4) solid electrolyte and Na3V2(PO4)3 electrode active material. By using the additional electrodes as reference electrodes, they could separately measure the potentials of the positive and negative electrodes during battery operation. Moreover, by using the additional electrodes as charge state adjustment electrodes for the positive and negative electrodes, they could measure the remaining capacity of the positive and negative electrodes and evaluate the reduction of carrier ions (ions that travel between the positive and negative electrodes through the electrolyte during battery charge and discharge) during degradation.
Furthermore, by using a multilayer special cell that incorporates symmetric cells made of the same material for both positive and negative electrodes instead of the battery, they were able to create symmetric cells in specific charge states (batteries using electrodes of the same state and same material for both positive and negative electrodes) and obtain electrochemical impedance information for specific electrodes in certain charge states. Next, they estimated, from the electrochemical measurement results, the causes of capacity degradation behavior that occurs when continuing charge-discharge cycles of the target battery.
The results revealed that the remaining capacity of the positive electrode after degradation was very large, indicating that carrier ions were lost in large numbers due to degradation. The loss of carrier ions that occurred in the target all-oxide solid-state battery was attributed to the inactivation of carrier ions when the solid electrolyte decomposes due to exposure to low potentials on the negative electrode side.
Fujihara commented: "I believe that 'evaluation' of storage batteries is essential for better utilization of storage batteries. Currently, various new types of batteries-not only existing lithium-ion batteries but also all-solid-state batteries-are being developed rapidly. Enabling proper evaluation of these various batteries makes it possible to accelerate development and achieve better utilization in society. I hope to continue contributing to storage battery utilization through the development of evaluation methods and evaluation itself."
Journal Information
Publication: ACS Applied Energy Materials
Title: Multilayer Cell System with Reference/Carrier-Ion Source Electrodes Elucidates the Degradation Mechanism Governing All-Oxide Solid-State Batteries
DOI: 10.1021/acsaem.4c03176
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




