Since ancient times, human beings have used their wisdom and technology to better understand the Earth we live on. For example, the deep interior of the Earth, which is hard to see directly, has been studied utilizing seismic waves and other techniques. Yet, it is still full of mystery.
In this context, a research group, including the University of Tokyo, has announced that experiments have shown a large amount of hydrogen in the central "core" of the Earth. The primitive Earth may have had 50 times as much water as today's oceans. They say that serial collisions of small celestial bodies brought a large amount of water, most of which got degraded, resulting in the hydrogen moving to the core. This outcome is vital in understanding how the current life-giving Earth was formed, like the ratio of land to sea and the depth of oceans, and so on.
Where did all the water go?
4.5 billion years ago, it seems like there were an order of magnitude more water on the Earth than there is now held in the oceans, which was brought here by celestial collisions. Clarifying what happened to the water is a crucial issue for understanding how the Earth was formed.
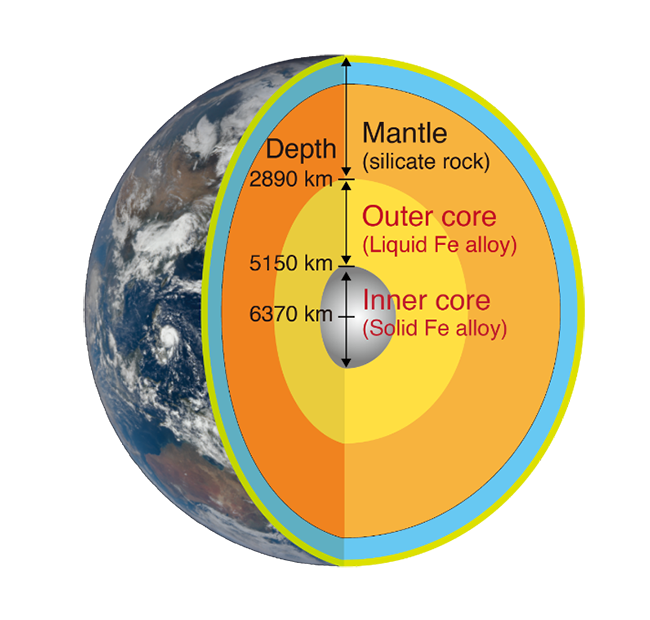
The main component of the core is iron, which comprises a solid "inner core" and a liquid "outer core." Since the density of the outer core is 8% lower than that of iron, it has been pointed out for about 70 years that it must contain a large amount of some light element.
The primitive Earth was covered with the "Magma Ocean," a sea of magma. The iron that fell here from celestial collisions did not melt but sank deep into the core because of its high specific gravity. In doing so, it is imaginable that the iron absorbed the light elements contained in the magma through chemical reactions. In addition to hydrogen and oxygen, which make up water, sulfur, silicon, and carbon were all considered "the light element" but it had not yet been clarified.
It is impossible to directly extract and examine the material in the outer core, which is at a depth of more than 2,890 kilometers. It was also technically difficult to reproduce in a laboratory the high-pressure, high-temperature condition of 50 gigapascals and 3,500 degrees Celsius at a depth of 1,200 kilometers, where the chemical reaction took place.
Reproducing high-pressure and high-temperature conditions in an experiment
In response to these issues, a research group led by Kei Hirose, a Professor (High-pressure geoscience) at the University of Tokyo's School of Science and the Tokyo Institute of Technology's Earth-Life Science Institute, used special experimental equipment. The diamond and laser setup enabled them to reproduce conditions of 30-60 gigapascals and 2,800-4,300 degrees Celsius. First, they focused on hydrogen and investigated how much it binds to magma or iron through chemical reactions. For the analysis, they used SPring-8, a large synchrotron radiation facility in Sayo-cho, Hyogo Prefecture, and an isotope microscope at Hokkaido University.
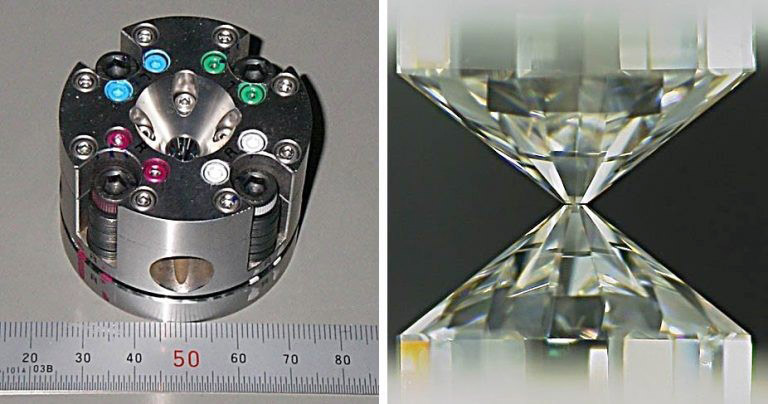
(Provided by the University of Tokyo)
Presently, you can find the water which didn't become hydrogen and remained in the Magma Ocean, within seawater and the rocks of the "mantle," the outer part of the core. That water had been confirmed to be at a concentration of about 700 ppm (1 ppm is one part per million) in the Magma Ocean era. After calculating together with their experiment results, they concluded that 3,000 to 6,000 ppm of hydrogen moved to the core.
Thus, they found out that there were between 30 to 70 times, or roughly put, 50 times, more water on the ancient Earth and that most of it was taken into the core as hydrogen. It is inferable that this phenomenon created the balance of sea and land as we see today, which has been nurturing our lives.
"What's the Earth Made of?" The Quest continues
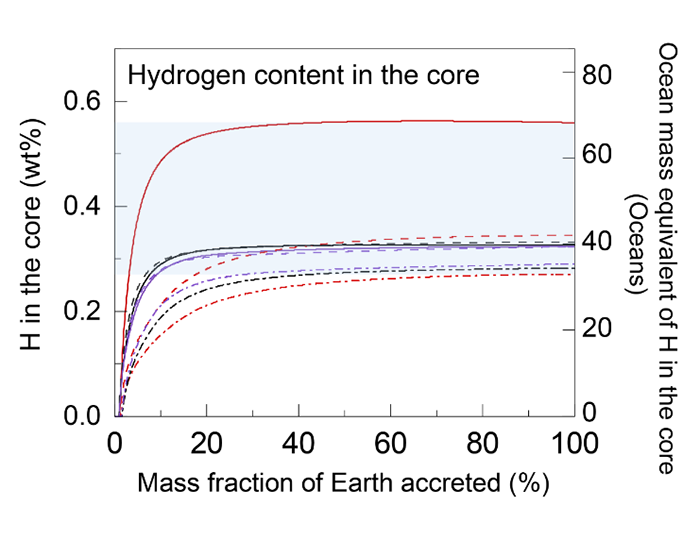
The simulation also revealed that even if the primordial Earth was 10% bigger than the present Earth, the hydrogen in the core is unlikely to increase any further. For example, the core of Mars, a rocky planet whose mass is about 10% of the Earth, is likely to have the same amount of hydrogen as the Earth's. That all fits with the story that Mars once had a rich ocean.
The research group consisted of the University of Tokyo, Tokyo Institute of Technology, Hokkaido University, and Japan Synchrotron Radiation Research Institute. The results were published in the British science journal Nature Communications on May 11.
Professor Hirose says, "The mass of the core is one-third of the Earth. It is a big problem that we haven't identified the light elements that occupy 20% of that mass because that means we don't know the material that created the Earth. We have found out that there is a large amount of hydrogen, but in the future, we would like to clarify the amount of other elements to clarify the whole picture of the core." It will help us to understand the formation process of planets, including those outside the solar system, and the conditions for the existence of extraterrestrial life.
What is the Earth made of? Our quest to answer this makes us reconsider the position we are in and expands our vision into space. There may not be as moving a drama as we saw with the asteroid probe Hayabusa but results worthy of passing on to the next generation are expected.

(Provided by NASA)
Original article was provided by the Science Portal and has been translated by Science Japan.




