There are currently approximately 422 million patients with diabetes worldwide. Complete recovery from the disease is difficult, and patients with diabetes require lifelong control of their blood glucose levels. One issue is that patients with diabetes can only accurately evaluate and determine the results of their efforts to control their blood glucose levels after undertaking a blood test at a hospital. In response to this, PROVIGATE (Bunkyo-ku, Tokyo) is working toward developing an inexpensive blood glucose sensor that can easily measure blood glucose levels at home. This sensor aims to prevent the onset and aggravation of diabetes by providing patients with the numerical results of their blood glucose levels at home, allowing them to conveniently continue their daily medical treatment. This blood glucose sensor will create a new step in changing the way we manage diabetes.

Weekly "quizzes" make it easier for patients to determine the results of their efforts
Glucose and other sugars in the food we eat are absorbed by the intestine and used as an energy source in the body. Insulin, a hormone secreted by the pancreas, helps cells absorb sugars and plays a role in maintaining blood sugar levels within a certain range. However, insulin does not work properly in patients with diabetes, resulting in chronically high blood sugar levels. If high blood sugar levels are left untreated, blood vessels may be damaged, leading to serious complications such as nephropathy, retinopathy, neuropathy, cerebral infarction, and myocardial infarction.
A big issue is that it is difficult for patients to be aware of the symptoms of type 2 diabetes, which accounts for the majority of diabetes cases, due to its slow progression and their first visit to a hospital for examination is often delayed. In addition, with a proper diet, exercise routine, and medication, patients with diabetes can lead a normal life similar to people who do not have diabetes; however, it is up to the patients to continue to make an effort. Due to these difficulties, even if patients with diabetes visit a medical institution for treatment, their disease conditions become severe in many cases. Koshin Sekimizu, CEO of PROVIGATE, has stated the structural limitations of the current medical care. He says, "Most diabetes patients receive a medical examination only once every 1-3 months, and they can review the results of their medical treatment only by a blood test. Also, most diabetes patients do not have a self-monitoring blood glucose meter, so they cannot grasp their daily condition. Even if they make efforts to control their blood glucose by themselves, it will be difficult to continue proper medical treatment without reviewing the results of blood sugar improvement numerically."
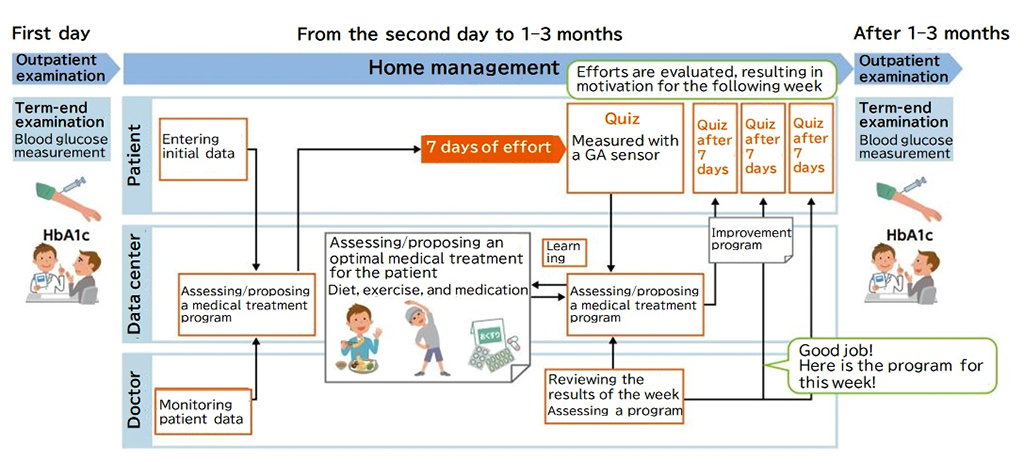
PROVIGATE is developing a sensor that is economically and physically less burdensome than previous methods, allowing any patient to easily grasp their blood glucose levels, and its application supports the prevention and treatment of diabetes (Fig. 1). The biomarker used by the sensor is glycoalbumin (GA), which is glucose bound to albumin, one of the proteins in blood. GA is a superior alternative blood glucose marker, which can indirectly measure 1-2 weeks of average blood glucose levels. Unlike a general self-monitoring blood glucose meter that requires blood sampling many times a day, this sensor determines the latest average blood glucose level by taking a measurement once a week.
The sensor also allows doctors to properly understand the condition of patients between examinations and provide a medical treatment program that suits the patient's life. Describing the benefits of the sensor, Dr. Sekimizu says, "The medical examination at the hospital is like an 'End of term examination' at school that takes place once every few months. We need to be well prepared to get results. On the other hand, an examination with our sensor is like a 'quiz' that dutifully reviews the recent efforts of patients. Their weekly efforts to eat in a well-balanced manner and exercise are reflected in the numbers. Patients can review the numbers on a weekly basis while their memory is still fresh."
Semiconductors as biosensors
From prototype to mass production
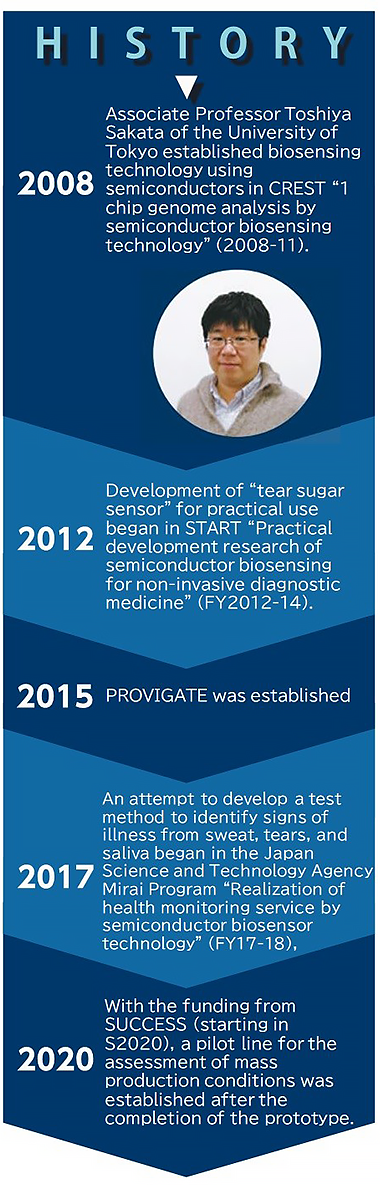
The development of the blood glucose sensor began with a biosensor developed in 2008 by Associate Professor Toshiya Sakata of the Graduate School of Engineering at the University of Tokyo. Because various components can be analyzed by manipulating the interface between the measurement sample and the semiconductor sensor, he considered putting a semiconductor sensor to practical use as a glucose monitoring device. In 2014, Dr. Sekimizu met Dr. Sakata, who had begun to move toward the commercialization of glucose monitoring with the support of START. Dr. Sekimizu says, "In our conversation, I was impressed with the core technology, and I thought it had great potential for measuring various biomarkers. In 2015, I founded PROVIGATE with Dr. Sakata and others."
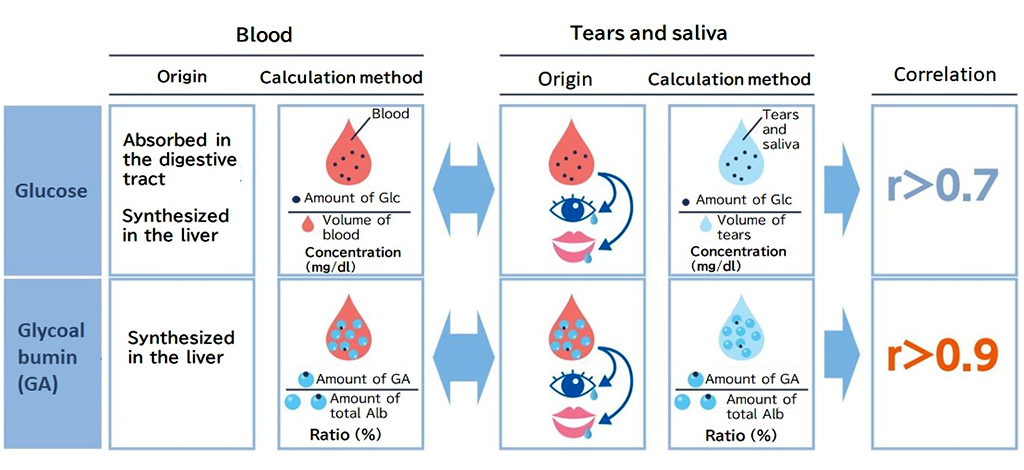
At the beginning of its founding, the team were working toward developing a tear sugar sensor; however, when analyzing the components of tears, they noticed that tears contained albumin. In addition, they found an extremely high correlation between the proportion of GA bound to sugar and the blood glucose level (Fig. 2). The team decided to use GA as a biomarker because the accurate measurement of blood glucose levels by collecting a small amount of blood once a week is more clinically useful than measuring its approximate value using tear sugar content.
Prototype verification has already been completed, and now the team are moving toward establishing a mass production system. The development of the GA sensor is led by Narushi Ito, CTO of PROVIGATE, who demonstrated continuous glucose monitoring in interstitial fluid for the first time worldwide and has experience in launching a digital urine sugar meter for home use. "Verification of the principle in the laboratory is not enough to make a product. Industrial mass production of a high-precision sensor requires manufacturing technology based on experience. Accuracy and stability cannot be achieved simply by imitating the conditions described in papers and patents. At PROVIGATE, engineers with experience in different fields of development gather for efficient development focusing on key points." he said with confidence.
Building a patent network in an oligopolistic market
The passion of developers is the driving force
While there are few successful medical device ventures in Japan, PROVIGATE is aiming for practical use of their GA sensor. Dr. Sekimizu cites the construction of intellectual property based on business characteristics as one of the factors. He says, "Unlike the monopoly market of therapeutics, diagnostics and diagnostic medical devices basically have an oligopolistic market where technical and patent monopolies cannot be easily developed, but manufacturing technology allows for a long-term business. In order to compete with competitors in the world, we need to build and maintain sufficient expertise and a patent network."
Dr. Ito adds that the passion of developers is the driving force. "There are regulations and rules in medical care to prevent accidents with which we must comply. Creating new products while complying with these regulations and rules provides a series of challenges. The passion of developers who best understand the technology to put it to practical use helps them overcome these hardships."
The team aims to obtain regulatory approval for the GA sensor as a measurement system for medical institutions in 2023, followed by its expansion as a medical device for home use. According to the International Diabetes Federation, three-quarters of patients with diabetes are concentrated in low- and middle-income countries. If patients with diabetes could safely keep track of their blood glucose levels at home at a low cost, it would be a great help to many patients, their families, and people in the preliminary group. The GA sensor developed by PROVIGATE will change the status of diabetes medicine worldwide.