It is estimated that one in two Japanese people will suffer from cancer at least once in their lifetime. Along with surgery and chemotherapy, radiation therapy is one of the major modes for cancer treatment. However, it is widely known that the more the tumor tissue grows, the less effective radiotherapy becomes. In 2006, Professor Emeritus Yasuhiro Ogawa of Kochi University developed the radiosensitizer "KORTUC," which is a mixture of hydrogen peroxide and hyaluronic acid. Since then, he has successfully demonstrated its high efficacy in clinical research. KORTUC Inc. (Minato City, Tokyo), a company established to develop the product and bring it to patients around the world, is currently running its clinical trial for locally advanced breast cancer with the UK Institute of Cancer Research. A radiosensitizer originated in Japan is about to bring a paradigm shift not only to radiation therapy but also to cancer treatment globally.

Current drug pricing system in Japan hinders development
Four years after the discovery of X-rays by Wilhelm Röntgen in 1895, X-ray irradiation began to be used for the treatment of skin cancer. Since then, radiation therapy has progressed greatly to become a powerful cancer treatment along with surgery and chemotherapy. Irradiation of lesions with strong radiations destroys cancer cells. However, the more the tumor tissue grows, its hypoxic level and the amount of antioxidant enzyme increases. Thus radiotherapy gets greatly impaired to about one-third of base efficacy.
In 2006, Professor Yasuhiro Ogawa of Kochi Medical School (at that time) succeeded in greatly improving the effectiveness of radiation therapy using hydrogen peroxide as a sensitizer, which can supply oxygen and deactivate antioxidant enzymes. This sensitizer, named "KORTUC," is a mixed solution of hydrogen peroxide, often used as a disinfectant, and hyaluronic acid, which has excellent water retention and viscosity properties which widely exists in human body. Although hydrogen peroxide gets decomposed into oxygen and water, being mixed with hyaluronic acid it gradually generates oxygen when injected into solid cancers and inactivates antioxidant enzymes. Since its approval by the Institutional Review Board of the Medical School, Dr. Ogawa has performed radiation therapy with KORTUC for various types of cancers, mainly breast cancer, and demonstrated its high efficacy.
With the hope of saving many patients, Dr. Ogawa consulted with Japanese pharmaceutical companies for the commercialization of KORTUC with the aim of putting it into practical use. However, he was unable to receive a positive response from any company. Kazuyuki Matsuda, president of KORTUC Inc., who is currently working on its clinical development as a drug, analyzes the reason as follows: "The expected price of KORTUC in Japan seems to be unattractive to pharmaceutical companies. This is because under the current Japanese drug pricing system, the price of KORTUC will be cheap, based on the cost of its raw material hydrogen peroxide rather than on its clinical value to transform a radiation therapy to "definitive treatment".
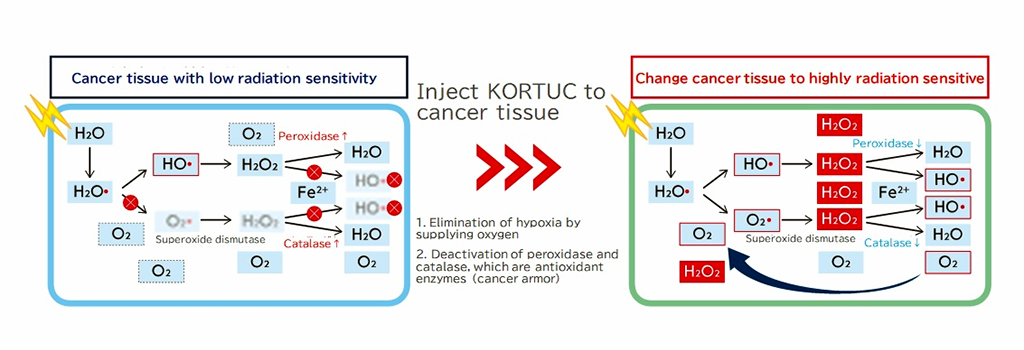
However, KORTUC, when injected into cancer cells, deactivates antioxidant enzymes and supplies oxygen to maintain the radicals produced by irradiation. Furthermore, excess hydrogen peroxide generates radicals in lysosomes to induce apoptosis, which enhances the cancer cell-killing effect.
To get regulatory drug approval in Japan, you must undergo clinical trials, which incur huge costs. Once approved, drugs are sold at a price determined based on various factors such as raw material cost, and the development cost is recovered from the profit. Under the current Japanese drug pricing system, the price of drugs made from cheap raw materials is set low irrespective of their effectiveness. Therefore, it is not surprising that Japanese pharmaceutical companies did not have any incentive to develop KORTUC even if it would have a huge potential as drug.
Proposal to conduct a clinical trial from the United Kingdom
Expected to launch the product in 2025
In 2015, almost 10 years after the implementation of KORTUC therapy in Japan, Dr. John Yarnold of the Institute of Cancer Research/the Royal Marsden Hospital in the United Kingdom who had a long-standing relationship with Dr. Ogawa, proposed that he conduct a clinical trial in the United Kingdom for the practical application of KORTUC therapy. In Europe, the proportion of radiation therapy is much higher than in Japan, and the price of a drug is determined according to their therapeutic effects. This means that drugs can be commercialized more readily in Europe by incentivizing pharma/medical players. Although Dr. Ogawa had already retired from the university, he began to look for people familiar with contracts and funding for clinical trials in the United Kingdom.
He was then introduced to Mr. Matsuda through a common acquaintance who was a venture capitalist. Looking back, Mr. Matsuda says, "When I was introduced to Dr. Ogawa, the efficacy of KORTUC had been confirmed in more than 700 cases, and I felt that it was promising." In 2015, he established KORTUC Inc. and started working toward its clinical development. However, it is difficult for a business to succeed simply by selling a mixture of hydrogen peroxide and hyaluronic acid. In addition, to be commercialized, hydrogen peroxide, which is reactive, must be stored and transported without being decomposed into oxygen and water. Therefore, Mr. Matsuda invited an expert to his team to study the formulation; this led to the development of the "prefilled injection kit" that was prefilled with a hydrogen peroxide solution.
In 2017, the phase-1 clinical trial for locally advanced breast cancer, which had been well treated by KORTUC therapy in Japan, began in the United Kingdom. In general, the safety and efficacy of new drugs are validated in clinical trials in three stages from phase 1 to 3. In phase-1 trials, safety is confirmed in a small number of patients. The therapeutic effects are investigated in detail, in addition to safety, in phase 2 and 3 on a higher number of patients. Mr. Matsuda explains, "Following our promising phase-1 results, the phase-2 trial is currently being conducted at five hospitals. In consultation with the Medicines and Healthcare Products Regulatory Agency of the United Kingdom, we are allowed to omit phase-3 trials if phase-2 result demonstrates good outcome." The UK outcome endorsed the clinical data for over 700 cases in Japan. If phase-2 results are good, KORTUC is expected to be launched in 2025.
Funds raised from VC
Bringing KORTUC to the world by expanding the scope of application
Despite the steady progress of the product and clinical development, Mr. Matsuda recalls the frequent struggles they initially had in terms of funding. Although KORTUC Inc successfully got a powerful clinical trial partner in the United Kingdom for a potentially global blockbuster, most of the Japanese venture capital investors were not enthusiastic about KORTUC and KORTUC Inc had difficulty raising funds. He recalls, "I signed the clinical trial contract in advance believing that funding would follow, although normally, people may think that the order is reversed. I wanted to bring excellent technology to the world as soon as possible. I searched around for funding opportunities and eventually got initial funding from a Japanese venture capital (VC) firm." With the aim to global expansion, they received funding from AXA, a major French insurance company, in June 2021, in addition to the funding from various agencies such as JST.
While clinical trials in breast cancer are advanced in the United Kingdom, they plan to expand the scope of its application to solid cancers other than breast cancer and potentially re-positions radiotherapy itself by becoming a definitive treatment in some of those indications. Moreover, given the unique product profile among cancer treatments, it can be broadly expanded to cover developing regions such as Asia and Africa in the future. To explore further opportunities, they are studying a combination of KORTUC with immunotherapy. If the steps progress in the future, the KORTUC therapy, which started from a local university in Japan, may bring paradigm shifts not only to radiation therapy but also to cancer treatment globally.
Therapeutic effects of radiation therapy are quite limited in hypoxic cancers because their exposure to radiation does not generate and maintain highly reactive radicals, which attack cancer cells (left). However, KORTUC, when injected into cancer cells, deactivates antioxidant enzymes and supplies oxygen to maintain the radicals produced by irradiation. Furthermore, excess hydrogen peroxide generates radicals in lysosomes to induce apoptosis, which enhances the cancer cell-killing effect.






