
As we move towards Expo 2025 Osaka, Kansai, Japan, new technologies ahead of the world are successively emerging from the Kansai region. One of these is a new cancer treatment called BNCT (Boron Neutron Capture Therapy), which started in May 2020. We asked Mitsunori Kirihata, Professor at the Research Center for BNCT, Osaka Prefecture University, and Tomoyuki Asano, Chairman of Stellar Pharma Corporation, about its development background. They contributed to the practical application of BNCT, which is expected to become the "fifth cancer treatment."
A cancer treatment with less burden on patients
Currently, there are four methods of cancer treatment in the medical field: surgery (surgical treatment), drug therapy such as anti-cancer drugs, radiation therapy, and immunotherapy. The treatment chosen is determined in consideration of the cancer type, the degree of progression, and the patient's condition, but there is always a need for more effective and less burdensome methods in the medical field.
Within this field, BNCT is drawing attention as a "fifth cancer treatment" that can expand the options for cancer treatment. BNCT treats cancer by administering boron drugs and using an irradiated neutron beam, which does not harm the body and can have a therapeutic effect in basically a "single (one day) treatment," placing less burden on the patient compared to other cancer treatment methods. This feature is due to BNCT's mechanism.
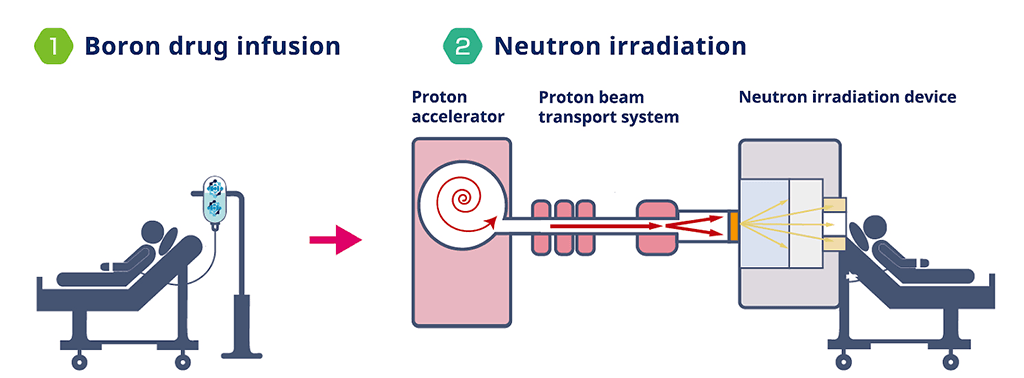
It treats cancer by administering boron drugs and irradiating them with a neutron beam.
(Source: Stellar Pharma website(https://stella-pharma.co.jp/en/))
The mechanism of killing cancer cells
BNCT is an abbreviation for "Boron Neutron Capture Therapy." As the name implies, two particles, boron, and neutrons play the "leading roles" in BNCT. In the treatment method, cancer patients are firstly given a boron drug consisting of a boron isotope (Boron-10) compound. This compound has the property of being selectively taken up by cancer cells. Then, when a weak neutron beam irradiates the diseased area, it hits the Boron-10 in the cancer cells and causes fission, producing radiation (alpha particles and lithium nuclei). This radiation destroys the DNA and mitochondria within the cancer cells, subsequently killing them. Assuming that a cell is about 20 micrometers, it is possible to destroy cancer cells with pinpoint accuracy since the generated radiation only travels about 10 micrometers.
Kirihata explains the features of BNCT, "Inside cancerous tissues, cancer cells don't exist in a homogeneous lump, but as a mix of cancer cells and normal cells. As a so-called 'cancer-cell selective therapy,' BNCT can kill cancer cells and minimize the impact on normal cells."
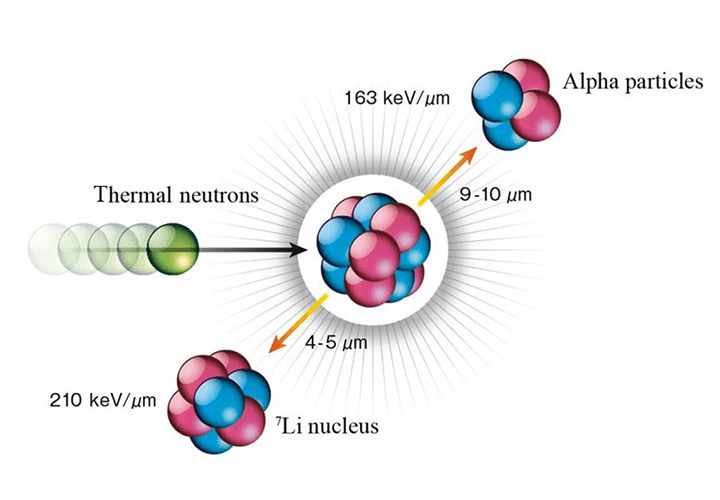
(Source: Japan Society of Neutron Capture Therapy website(http://www.jsnct.jp/e/index.html))
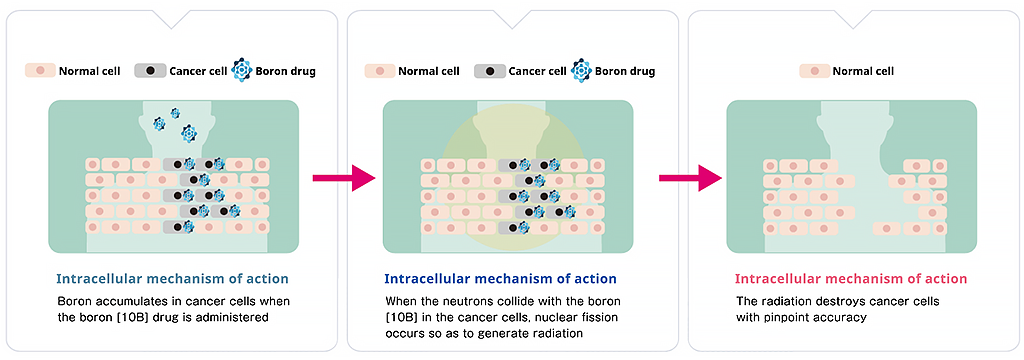
(Source: Stellar Pharma website(https://stella-pharma.co.jp/en/))
The Beginning of Boron Drug Development
The development of the boron drugs used for BNCT began with the encounter between Kirihata and Asano.
In the late 1990s, Stella Chemifa, a chemical manufacturer based in Osaka Prefecture, developed its own Boron-10 enrichment technology and started selling it as a neutron absorber for nuclear power generation. Asano, who was working in the company's research laboratory, was assigned to develop further applications for Boron-10.
"The enrichment plant for Boron-10 is located in Izumiotsu City (Osaka Prefecture). There was a nuclear reactor laboratory owned by Kyoto University in nearby Kumatori City, and I went there thinking that they might want Boron-10 from us."
After investigating, Asano learned that Boron-10 was already used in BNCT research.
"I found out that the boron drug for BNCT was being developed by a person named Dr. Kirihata at Osaka Prefecture University, which is very close to our company, so I immediately swung by his laboratory."
Kirihata was taken aback by Asano's sudden visit, as he had never dreamed that there would be an enrichment plant for Boron-10, a difficult to obtain material, right by his university. Until then, Kirihata had been importing Boron-10 from an American company, but slow delivery times and the high price made it difficult to proceed with his research.
"When I asked Mr. Asano how much Boron-10 his company was producing, he replied, 'We have a plant that produces Boron-10 by the ton,' which surprised me even more because until then, we had been importing a few dozen grams at a time." Asano recalls, "Dr. Kirihata asked me if he could have around 10 grams. I said, 'It may be a lot, but I'll bring one kilogram.' "

Stella Pharma Goes Independent
About half a year later, an industry-academia collaboration organization started to develop BNCT, and Asano undertook the development of BNCT boron drugs. BNCT was gradually gaining recognition, and the path to the development of boron drugs became clear. Around that time, he learned that Sumitomo Heavy Industries, Ltd., conducting research and development with Kyoto University, had begun full-scale development of a neutron generator to be installed in hospitals. It was a small cyclotron-type accelerator, and if completed, it would make it possible to introduce BNCT to ordinary hospitals.

Asano says, "That was a big 'breakthrough' for us." Stella Pharma was then spun off from its parent company, Stella Chemifa, to focus on BNCT boron drugs.
Development of pharmaceuticals for the benefit of people and society
As a result of this, the development of BNCT boron drugs started, but at that time, there was no precedent for the use of boron in cancer treatment, let alone for BNCT. It was necessary to verify "everything from scratch" through discussions with the Ministry of Health, Labour and Welfare (MHLW) and the Pharmaceuticals and Medical Devices Agency (PMDA), who approve pharmaceuticals and medical devices under the MHLW. "For example, in the case of cancer drug development, we could refer to what major pharmaceutical companies had done before, but in the case of BNCT, there was no precedent at all, so we needed to conduct thorough clinical trials," recalls Asano.
In general, it costs between ten to a hundred billion yen to develop a single anti-cancer drug. There was a fear that the development of the unprecedented BNCT boron drug would be even more costly. It was tremendously risky for a small company with a few dozen employees to enter the pharmaceutical industry for the first time. The company could have abandoned development at any time, but with the help of various measures, they were able to pass the approval process, which usually takes 12 months, in six months.

Another great aspect of support in the development process was the boost from Ms. Junko Fukada, the then chairperson of Stella Chemifa, the parent company. Fukada's belief that "Doing something for the world and people will eventually lead to profit for the company" backed them all along. The two say their feelings of "We want to save people who have cancer. We will develop products for the benefit of people and society," heightened even further.
Making a New Light for Those Fighting Cancer
So, with various boosts from collaborators in industry, academia, and government, Kirihata and Asano steadily progressed toward the significant goal of realizing BNCT. And finally, in March 2020, the BNCT boron drug, together with the cyclone accelerator, obtained pharmaceutical approval targeted for head and neck cancers (face, mouth, nose, throat, etc.). Later in May, the drug became covered by health insurance. BNCT treatment for ordinary patients started at the Southern Tohoku BNCT Research Center in Fukushima Prefecture and the Kansai BNCT Medical Center of Osaka Medical and Pharmaceutical University in Osaka's Takatsuki City. Both centers have received inquiries from all over Japan, and the number of treatments is steadily increasing.
Asano focuses on further expansion of BNCT, saying, "Next, we would like to expand the scope of treatment to include brain tumors and increase the number of medical institutions where BNCT is available." Kirihata is working on research that goes even further, "At present, we only have one type of medication, but in the future, we would like to realize 'tailor-made medicine' to make boron drugs according to the characteristics of each individual."
"We want to save as many people as possible by giving hope to those who have given up without being able to be treated. We hope BNCT will be a new light for people fighting cancer." With this strong desire in their hearts, their quest will continue.
(Provided by Sumitomo Heavy Industries, Ltd. and Osaka Medical and Pharmaceutical University)

Mitsunori Kirihata
Professor (Director) at Research Center for BNCT, Osaka Prefecture University. Ph.D. in Agriculture from Osaka Prefecture University. Having retired in 2012 after working as a professor at the Graduate School of Agriculture and Biological Sciences / Life and Environmental Sciences, Osaka Prefecture University, he assumed his current position as a project professor of Research Center for the 21st Century and Organization for Research Promotion.
Tomoyuki Asano
Chairman & CEO of Stella Pharma Corporation. After graduating from the Graduate School of Applied Chemistry, Kansai University, he joined Hashimoto Kasei (now Stella Chemifa) in 1996. Having engaged in the company's R&D work. In 2012, he became President of Stella Pharma, established in 2007, and assumed his current position in June 2020.
Original article was provided by the Science Portal and has been translated by Science Japan.




