After developing a process to synthesize methanol from methane, which has long been regarded as a "dream reaction", Professor Tsuyoshi Inoue of Osaka University Graduate School of Pharmaceutical Sciences helped launch a consortium to promote utilization of the process. There are companies in a variety of fields participating in the project and the applications are expanding.
The application of "cryo-electron microscopy," which is used for three-dimensional structural analysis of proteins, to sample preparation is attracting worldwide attention. The "dream reaction" is about to bring innovation to all industries.

Synthesis of methanol from methane
A great discovery that rewrites textbooks
In order to reduce real carbon dioxide emissions to zero by 2050, which will help achieve carbon neutrality, all industries in Japan are under pressure to save more energy. In the chemical industry, many of the reactions used to process raw materials can only be performed under high temperature and pressure conditions. Performing these reactions at ambient temperature and pressure would significantly reduce energy usage and so new catalysts and reaction conditions are being investigated.
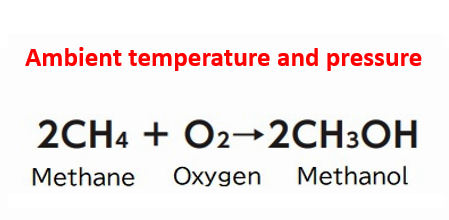
One of these reactions is the synthesis of methanol from methane. Methanol is currently produced by reacting natural gas-derived carbon monoxide with hydrogen at a high temperature of about 1000 °C. The main applications so far have been in adhesives, paints, synthetic resins and fibers, and pharmaceuticals, but in recent years, it has also attracted attention as a source of hydrogen for fuel cells. The conversion of gaseous methane and hydrogen to liquid methanol is also expected to simplify transportation and handling of the material. The production of methanol under ambient temperature and pressure conditions would greatly accelerate movement towards a hydrogen-based society.
Professor Kei Ohkubo of the Osaka University Institute for Advanced Co-Creation Studies has developed an oxidation reaction that uses chlorine dioxide (ClO2) and light to catalyze the synthesis of methanol from methane at ambient temperature and pressure. With this revolutionary oxidant at its center, the OPERA "Co-Creation Consortium for Oxidation Control" aims to efficiently carry out various reactions to facilitate their industrial utilization (Fig. 1). Professor Tsuyoshi Inoue of Osaka University Graduate School of Pharmaceutical Sciences, who is the OPERA Consortium Manager, explains the impact of this development: "This reaction appears simple at first glance, however, many chemists have attempted and failed to complete the reaction, and it has been regarded as one of the 'dream reactions' that chemists wanted to realize in the 21st century. It's a great discovery that rewrites the textbooks, so to speak."
Spark was corporate discussion
The key was to combine an oxidizer and light
The great discovery was sparked by a doctor's observation that using a disinfectant deodorant in Japanese aircrafts and hotels stopped the progression of disease and the fortuitous decision of the doctor to discuss this observation with Osaka University, with the aim of applying to pharmaceutical products. Professor Ohkubo was initially skeptical, but the group eventually discovered that chlorine dioxide which is the main agent of sterilization and deodorization in water, and can be supplied through chlorite ions, is an active species.
Professor Ohkubo, who specializes in photochemistry, released this component as chlorine dioxide gas under acidic conditions and exposed it to light. He then noticed that the chemical structure of chlorine dioxide changed significantly and that it had separated into active oxygen species and chlorine radicals. "The observation inspired me to think that, if I reacted chlorine dioxide with methane, chlorine radicals would extract a hydrogen atom from the methane, and active oxygen would bond to it, which could possibly produce methanol", said Professor Ohkubo.
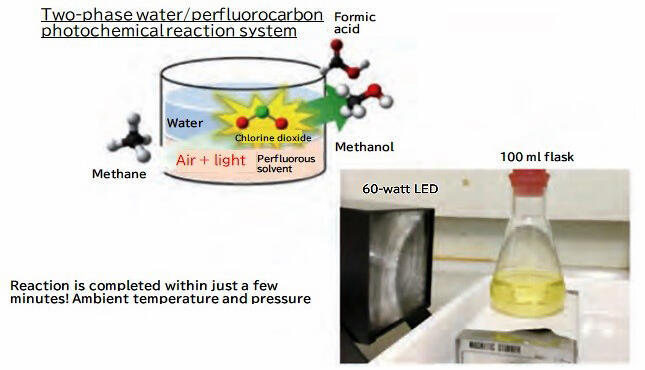
However, when the reaction is performed in an organic solvent, the highly reactive radicals react with the solvent and cannot be selectively reacted with methane. Therefore, Professor Ohkubo decided to perform the reaction in a two-layer solution of aqueous chlorine dioxide and methane dissolved in a perfluorinated solvent, which is exclusively composed of carbon and fluorine atoms. When the flask that contained the solution was exposed to light from a 60-watt LED bulb for several minutes, the methane and chlorine dioxide reacted, and methanol and formic acid (HCOOH) were generated under ambient temperature and pressure conditions (Fig. 2). "The reaction initially produced 14% methanol and 85% formic acid, but we are currently investigating conditions that will allow us to synthesize methanol more selectively," says Professor Ohkubo.
Professor Inoue thought that if other substances could also be oxidized under ambient temperature and pressure conditions by irradiating chlorine dioxide with light, then the process could be applied to a variety of industrial fields, including energy production, as well as to the medical and pharmaceutical fields. Motivated to use this great discovery for the benefit of society, Professor Inoue, along with Professor Ohkubo and Associate Professor Haruyasu Asahara of Osaka University Graduate School of Pharmaceutical Sciences (at that time, Kochi University of Technology specially appointed lecturer) have noted a variety of potential applications. "There were some ideas that aren't feasible in retrospect, but at that time, my dream kept expanding and I would think that we could try something like 'this' or 'that'," said Professor Inoue with a smile.
The ideas at this time led to the application to OPERA aiming for technological innovation through industry-academia collaboration. The group approached a candidate company to participate in joint research with OPERA and obtained consent immediately. "Many joint research courses have been set up at Osaka University, and we have actively participated in industry-academia collaboration here. Some examples include having a researcher from a company resident at the university and promoting joint research. There was a local advantage and a community of people established here to help promote research. In this way, this OPERA project, led by Professor Inoue, was launched.

A demonstration experiment started in Hokkaido with the aim of creating circular dairy farming
OPERA recommends developing new partnerships to promote open innovation based on the core technologies it has developed. One of these is in Hokkaido's Okoppe town, where Osaka University has signed a partnership agreement. Okoppe faces the Sea of Okhotsk and has a population of about 3700. Dairy and fishing are the core industries there, and the town contains a milk cow population of around 11,000, which is about three times the size of the human population. "The start of this collaboration was that the mayor of Okoppe town, Mr. Kazutoshi Hazama, wrote to me. The mayor was originally a dairy farmer, and he was highly aware of environmental issues. By using an English/Japanese dictionary, he had even read an article that I had written in English. I was impressed with his enthusiasm," recalls Professor Ohkubo.
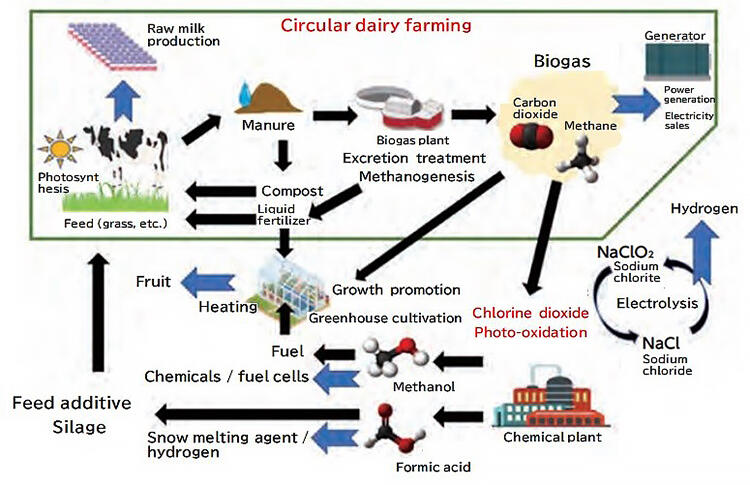
In Okoppe town, the processing of milk cow manure, which is generated in large quantities every day, is an issue for dairy workers, as well as for the entire town (Fig. 3). The methane present in the biogas produced by fermenting the manure is used as fuel to generate electricity, which is then sold to Hokkaido Electric Power Co., Inc. However, the processing plant can only accommodate the manure of 560 milk cows, a mere 5% of the town's cow population, and there is a limit to how much it can be used as electricity. This led to discussions regarding the fact that Okoppe wanted to efficiently utilize methane from biogas.
The annual supply of methanol in Japan is about one million tons, and it is mainly imported from the Middle East and United States. Meanwhile, the annual supply of formic acid is about 10,000 tons, most of which is imported from China. Most of this formic acid is used in Hokkaido to ferment grass for milk cows, fertilize crops, and melt snow at airports. Similar to methanol, formic acid is attracting attention as a source of hydrogen and as a hydrogen carrier for a hydrogen-based society, but high production costs have limited its applications thus far.

Professor Ohkubo estimated that biogas from 560 animals could be processed using photo-oxidation to produce 80 tons of methanol and 400 tons of formic acid per year. If biogas processing plants could be established across Hokkaido and accommodate manure and biogas from 1.35 million heads of milk cow, 200,000 tons of methanol could be produced annually, thereby fulfilling 20% of Japan's annual imports. This would also increase the annual production of formic acid to one million tons, allowing Japan to become an exporter of the product. There is no doubt that dairy farmers would benefit greatly if manure processing, which has historically been a costly endeavor, became profitable.
Inspection teams from all over the world, not just Japan, are visiting this Okoppe initiative. It has been reported that there are proposals to apply the technology to other types of livestock, as well as to waste treatment facilities, and Professor Ohkubo is feeling the positive impacts of the high degree of attention. Professor Ohkubo speaks passionately about his dream: "If small-scale biogas plants could be built in different places, we will be much closer to the realization of a sustainable society. Ultimately, I would like to build a carbon-neutral circular dairy system" (Fig. 4).
Technology also applied to cryo-electron microscopy
High resolution with easy sample preparation
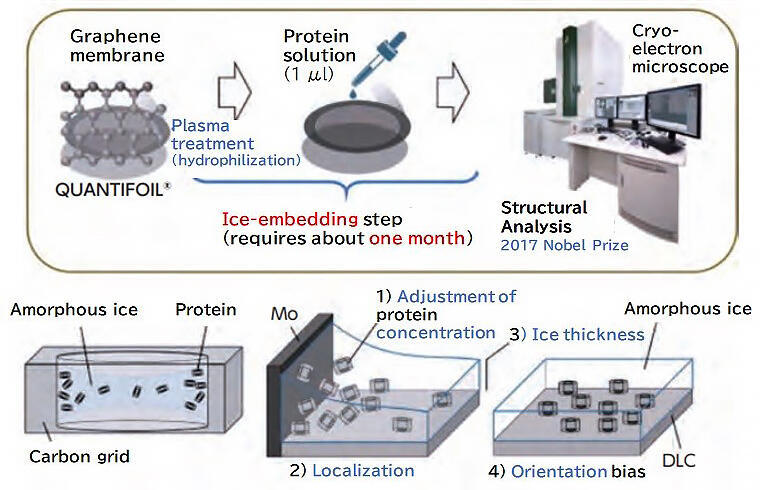
Professor Inoue, who specializes in biochemistry, wanted to use the oxidation reaction Professor Ohkubo discovered to prepare samples for cryo-electron microscopy, which has recently been frequently used for protein structural analysis. Even though cryo-electron microscopy is convenient because biomolecules can be observed without crystallization, sample preparation, which requires proteins to be dispersed in thin ice, is quite difficult, and the processing of individual samples often takes about a month (Fig. 5). Researchers have developed methods for laying a single-layer film of carbon molecules (i.e., graphene) under the sample and for temporarily hydrophilizing the surface of the graphene using plasma treatment, in order to increase the affinity between the graphene and proteins. However, this method was not stable, and it was difficult to immobilize the protein the in various directions required for thorough examination. "Graphene is an extremely stable molecule that is difficult to chemically react with. I thought that if we applied the photo-oxidation reaction developed by Professor Ohkubo, we could introduce oxygen functional groups on graphene, allowing it to bind proteins directly after chemical modification," says Professor Inoue.

Dr. Asahara recalls that it was difficult to control the photo-oxidation reaction and that it was very difficult to determine optimum reaction conditions. However, after trial and error, he succeeded in photo-oxidizing graphene in 2020. Dr. Asahara developed a graphene thin film using a compound that binds to proteins more readily. By simply dripping a solution of dissolved protein molecules onto the thin film and then washing and freezing the film, the proteins are neatly lined up on the graphene film in various orientations, up, down, left, and right, with no single molecule overlapping (Fig. 6). "The binding of proteins in different orientations is extremely important for structural analysis, and the three-dimensional structure of proteins can be determined using images acquired from different orientations."
Dr. Asahara describes the result as follows: "Sample preparation is easier than ever and can be performed by anyone in just 10 minutes. It is easy to adjust the focus of the image, and it is now possible to simultaneously capture images of molecules in various orientations, as is necessary for three-dimensional structure analysis. As a result, significantly fewer images are necessary, and the process has become so efficient that more computers are needed so that data analyses could keep up with the data measurement." The significant improvement in the efficiency of protein structure analysis is expected to reduce the development period of drug discovery. In the future, Dr. Asahara plans to take on the challenge of developing graphene sheets that are suitable for membrane proteins, which are more difficult to analyze in terms of three-dimensional structure.
A wide range of applications: 70 companies participate and put discoveries into practical use
The Oxidation Control Co-Creation Consortium is achieving its goals, one after another, at a speed that greatly exceeds their initial expectations. At Osaka University, medical, dental, and pharmaceutical industry collaborations with the medical, dentistry, and pharmacy schools are progressing, but Professor Inoue talks about the merits of conducting research at OPERA. "University professors usually perform research individually, according to their own interests, but in the field of industry-academia collaboration such as 'OPERA', great power is created by working together as a team towards a single goal: to solve the various problems that society needs to solve. OPERA will lead to social implementation of discoveries that cannot be achieved by universities alone. Timely communication and targets bring a pleasant tension to the research process. There are six companies participating in research, but the number of companies that cooperate in social implementation has increased to 70, and their responsibilities are significant. " In November 2020, the Japan MA-T Industrial Association was established with a focus on companies interested in photo-oxidation technology.
Professor Ohkubo notes that collaboration between companies is a key to success. He says, "The gathering of companies with strengths in their respective fields under the goals set by OPERA has enabled the formation of cross-functional teams, and I feel that the entire project has come together nicely." Dr. Asahara also says that photo-oxidation is a technological 'seed' with wide-ranging applications that have been successful. "We alone cannot cover all the applications, since they are too wide in scope. With the consortium-type OPERA project, researchers and companies can increase participation and expand collaboration. Every day is very exciting, and I enjoy each research subject," he says with a smile.
In April 2022, the Education and Research Building of the Graduate School of Pharmaceutical Sciences will be completed on the Osaka University Suita Campus. There is enthusiasm to further develop OPERA by expanding its education and research programs. Professor Inoue and his colleagues will continue to explore the great potential of the dream reaction and to make strong strides toward the realization of a sustainable society.
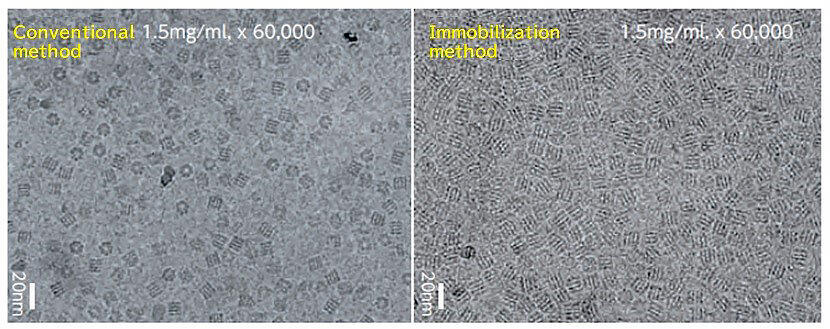
When compared with the conventional method, the immobilization method adheres the protein more uniformly. The effectiveness of the captured particle image is overwhelmingly high at about 90% with the immobilization method, compared to about 20% with the conventional method. (nm in the photo is a nano (10-9) meter)




