
Research and development for a decarbonized society is attracting attention as a necessary solution to the social issues we will face as a society in the future. We asked Akira Isogai, Professor at Graduate School of Agricultural and Life Sciences, the University of Tokyo, and Akira Oda, Assistant Professor at Graduate School of Engineering, Nagoya University, about their research and the prospects for a decarbonized society.
Cellulose nanofibers are comprised of plant fibers
Since the Industrial Revolution, humans have built affluent lives using fossil fuels such as coal and oil. On the other hand, this development has caused various problems, such as accumulated non-degradable garbage, marine microplastic problems, abnormal weather, and global warming. And now, there is a need to build a decarbonized society. A decarbonized society is a society where we reduce the emission of greenhouse gases such as carbon dioxide (CO2) and recover the emitted CO2 to nullify greenhouse gas emissions in real terms.
Isogai has developed a technology to produce cellulose nanofiber (CNF), a plant-derived material that can contribute significantly to the decarbonization of society. It is three nanometers thick (a nano is one billionth), only 1/30,000th of a hair. Despite being that thin, cellulose is very strong and can be chemically given various functions, drawing attention as a new industrial material.
"Cellulose is made up of linear chains of glucose, which is produced by photosynthesis using CO2 absorbed from the atmosphere. It accounts for about 40% of the weight of trees and is the most abundant polymer stored on earth. Since it is plant-derived, it can be reproduced, and even if incinerated after use, it does not increase CO2 in the atmosphere. Cellulose is a material that can contribute to a decarbonized society if it replaces plastics and other materials derived from fossil fuels," says Isogai.

Industry-academia collaboration will expand practical applications
Isogai had been studying the use of cellulose since he was a graduate student, but it was not easy to extract cellulose fibers. A turning point came in 1995. A Dutch research group reported their study results. The group used a substance called TEMPO (organocatalyst) as a catalyst to oxidize starch at standard temperature and pressure without using organic solvents. Isogai thought that the TEMPO catalyst could be applied to cellulose, a polysaccharide like starch, and worked to research his idea. In 2006, he and his graduate students succeeded in developing technology to obtain CNF by treating pulp fiber with TEMPO catalyst without using large amounts of energy or harmful chemicals.
He took the result to the industrial world. Then, in addition to the merits of contributing to global warming countermeasures, the ability to add a variety of functions drew attention, which drove many companies to enter industry-academia collaborative research and development. CNF production methods other than TEMPO catalysts also progressed, with some already being put to practical use.

One example is paper diapers for nursing care with metal ions that have deodorizing functions attached to their CNF. Ballpoint pens that allow us to write without applying pressure are also commercially available ― by mixing CNF as a dispersant, the ink becomes homogenized and smooth. It is also used as an admixture in concrete, shampoo, rinse, etc.
In addition, a film that is impermeable to oxygen has been developed by blending CNF into it. If CNF is applied in packaging materials, deterioration of food and medicine due to oxidation can be reduced. Since we consume a massive amount of packaging materials derived from fossil fuels, even if CNF replaces only some of them, CO2 emissions will decrease considerably.
Isogai is also looking at the application of CNF to tires. Soot (carbon black) is added to tire rubber to increase stability, but as tires wear away gradually, the carbon black is discharged into the environment. In that regard, CNF will not negatively impact the environment even if it is released as the tire becomes worn. "CNF and rubber go well together and increase strength when they are mixed. Automobile tires using CNF have already been developed and are on the market, becoming a promising alternative candidate for reducing their environmental impact."
Utilizing the advanced technologies of Japan's paper industry
At present, CNF is still expensive due to its low production volume, and its use is limited to applications that bring high functionality with little added volume. In the future, if manufacturing it at low cost and in large quantities becomes possible, the range of applications will surely expand. In this way, we hope to replace petroleum-derived materials with CNF even if it is little by little.
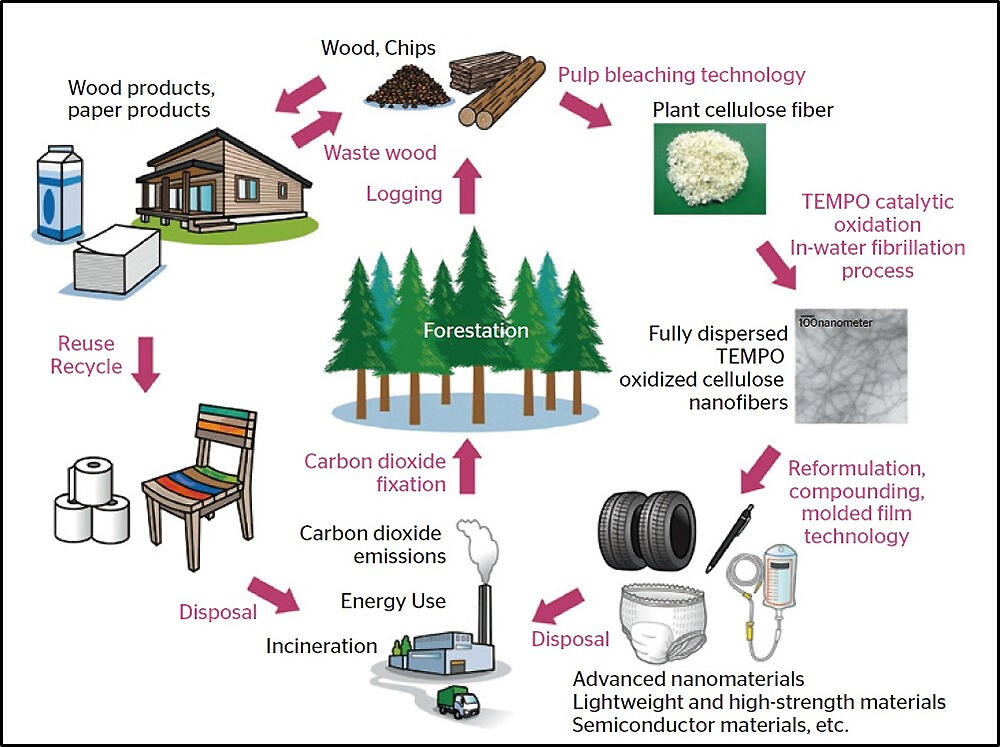
The Japanese paper industry has advanced technology to create high-quality papermaking pulp and electricity. "There is a lot of unused thinned wood in the mountainous areas of Japan. By making use of it, we will be able to revitalize the forest industry in Japan. Although there are some problems, we have raw materials and technologies. If we can create a new biomass-derived sector in Japan, we will contribute to the global issues we face," says Isogai. Because of its wide range of applications, contributions to the various goals of the SDGs are also expected.

Aiming for decarbonization by realizing "Fairytale Reaction"
Meanwhile, Oda is conducting research that will help contribute to energy issues.
The amount of available natural gas reserves, which are currently used as gas in homes, is increasing due to advances in mining technology. Methane, the main component, produces less CO2 when burned than oil or coal and is getting a lot of attention as a clean fuel. However, because methane is a gas at ordinary temperature and pressure, transportation and storage costs are high. So, converting it to methanol, a liquid at ordinary temperature and pressure, has been a requirement.
On the other hand, the reaction to convert methane into methanol has long been called a "fairytale reaction" among chemists. Oda explains the reason for this. "The only difference between methane (CH4) and methanol (CH3OH) is the presence or absence of one oxygen atom. To convert it to methanol, just one oxygen atom addition to methane is necessary, i.e., partial oxidation. However, methane is a very stable substance with strong bonds between atoms, and it takes a lot of energy to break the bonds between hydrogen and carbon. Despite this, when energy is applied, methane is completely oxidized to CO2 and water."
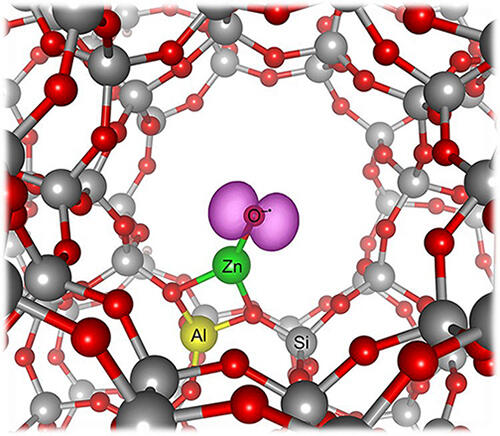
Oda and his research group have discovered "oxyl," active oxygen which can partially oxidize methane at low temperatures to prevent the reaction from going too far. Oxyl is in a state where it can receive electrons easily and is therefore easily incorporated into the CH bonds of methane. Oda and his colleagues succeeded in producing oxyl using a porous zeolite catalyst. And they used it to synthesize methanol from methane at room temperature.
The wide practical application of this technology would enable efficient transportation and storage. Oda points out additional advantages to directly converting methane to methanol, which has a greenhouse effect 25 times greater than CO2. "Methanol is easy to handle as a chemical feedstock and can be used as a raw material for various substances. Methane has been used almost exclusively as a fuel because it is very stable as a substance and difficult to handle. But if it can be converted to methanol, it will have a much wider range of uses as a fuel and as a chemical raw material, just like ethane and propane."
Developing technology for low concentration CO2 capture
Oda and his colleagues have also developed a CO2 adsorbent by placing calcium ions on zeolite. They have confirmed that it can selectively adsorb CO2 even from gases containing CO2 at concentrations as low as 400 to 5000 ppm (ppm is a concentration unit indicating parts per million).
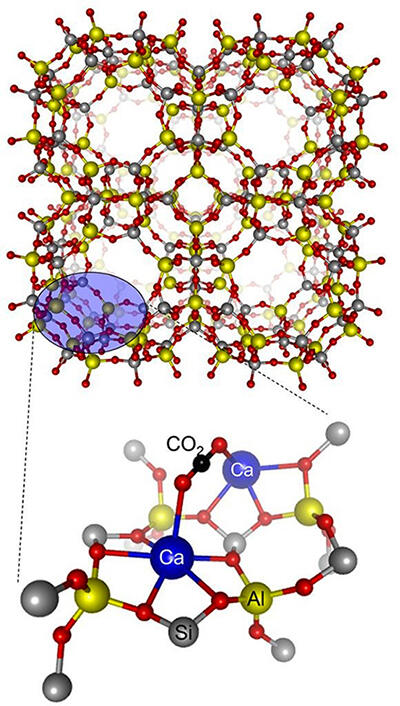
"It is difficult to stop all CO2 emissions, and in the future, technology to remove CO2 directly from the atmosphere will be required. It is not easy to collect CO2 that has diffused into the atmosphere, but if we can establish a technology to concentrate the diffused CO2, it will collect CO2 and be used in a wide range of ways. I believe that the development of CO2 adsorbents will lead to technology that can directly remove CO2 from the atmosphere," Oda expects further advances.
Oda had discovered the phenomenon that led to the creation of oxyl when he was an undergraduate student. However, he was told that the phenomenon was interesting but unbelievable. He was frustrated that he couldn't explain it well. "I wanted to convey the message more clearly, and to do so, I had to understand it better, so I did my best to research it. But before I knew it, I had completed my doctoral program (laughs). After that, as I combined experiments and calculations in my research life, I solved the mystery like unraveling a string, which led to this discovery."
Some experts from early on highly evaluated Oda's research. In 2013, when he was a graduate student, he was awarded the Nishina Prize, which recognizes outstanding graduates (or soon to-be) of science and engineering graduate schools in Okayama Prefecture. He was selected as a PRESTO (Precursory Research for Embryonic Science and Technology) researcher by JST. When he heard about the partial oxidation of methane, Oda was shocked to learn that the "Fairytale reaction exists!" This discovery is the fruit of his desire as a student to be able to create chemical reactions that a high school student could write but cannot realize. With this as a first step, he hopes to further contribute to constructing a decarbonized society.

Akira Isogai
University Professor, the Graduate School of Agricultural and Life Sciences, the University of Tokyo
Received Ph.D. in 1985 from the Graduate School of Agriculture, the University of Tokyo. After working as a postdoctoral fellow in the Department of Chemistry at the Institute of Paper Chemistry, USA, and as a professor at the University of Tokyo, he was appointed as the current position in 2020. Honorary Academic Doctor of Aalto University, Finland.

Akira Oda
Assistant Professor, Graduate School of Engineering, Nagoya University
Completed a doctoral course at the Graduate School of Natural Science and Technology, Okayama University, in 2015. After working as a visiting researcher (JST Sakigake researcher) at the Graduate School of Natural Science and Technology, Okayama University, he assumed his current position in 2019.
Original article was provided by the Science Portal and has been translated by Science Japan.




