
Proof of commonality in mammals a cause of excitement in the field
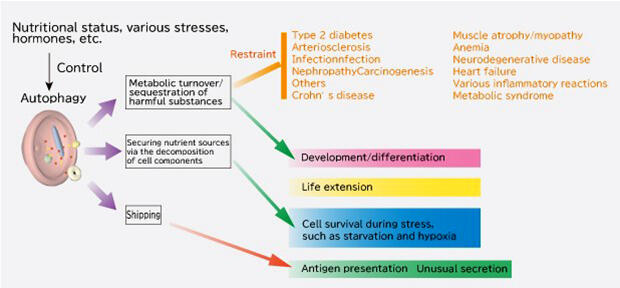
Autophagy research is increasingly expected to be applied to the treatment of a variety of diseases as the mechanisms underlying the process and their related genetics become apparent. Professor Tamotsu Yoshimori of Osaka University' Graduate School of Medicine/Graduate School of Frontier Biosciences is trying to bridge the gap between the results of basic research on autophagy and its practical application. Currently, he is the research director of the "Response to and formation of extracellular fine particles by autophagy" project as part of the CREST research area "Elucidation of biological mechanism of extracellular fine particles and the control system".
Dr. Yoshimori, who has been studying mammalian cell biology since he was a graduate student, is said to have started researching autophagy after being invited by specially appointed Professor Yoshinori Ohsumi of Tokyo Institute of Technology. "At that time, Professor Osumi had just decided to set up a laboratory at National Institute for Basic Biology, National Institute of Natural Sciences, and invited me as an assistant professor. I was in my thirties; I was looking for a research theme that would be my life passion. After hearing the story, I realized that there was real value in the research field."
At that time, Dr. Ohsumi had just discovered 14 genes related to autophagy in yeast, but this finding had not yet led to great excitement in the field. However, he was convinced that this phenomenon was fundamental to living organisms, and he invited Dr. Yoshimori, who was studying mammalian cells. This expanded the scope of Dr. Yoshimori's research from yeast to mammals. This arrangement was a perfect fit, and in 2000, Dr. Yoshimori discovered a protein related to autophagy in mammals. He showed that the basic mechanism of this process is an intracellular purification/recycling system common to eukaryotes.
Dr. Yoshimori discovered LC3, an autophagosome membrane-bound protein equivalent to the Atg8 protein found in yeast. It was also revealed that LC3 is lipidized by other autophagy-related protein groups and can be stably present in autophagosomes. Currently, it is widely used as a marker for detecting autophagy worldwide, and it has played a role in popularizing autophagy research.
Identification and elimination of harmful substances and relation to immunity
Another of Dr. Yoshimori's important findings is that autophagy eliminates pathogenic bacteria (Fig. 1). At the time his research began, the function of protein decomposition to secure nutrition under starvation conditions was known, but this discovery revealed that it also has a function related to immunity. Further research revealed that, as well as bacteria, it also selectively eliminates substances harmful to various cells.
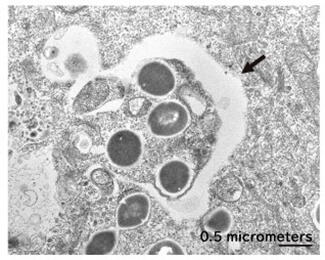
In general, substances from outside the cell are taken up by intracellular organelles called endosomes and sorted. Pathogens are also taken up by endosomes, but they try to break through the membrane and escape to the cytoplasm or puncture them to deliver their own proteins. After closely observing this phenomenon, Dr. Yoshimori found that damage to the endosomal membrane that encloses the bacteria causes a protein modification called ubiquitination, resulting in Atg protein accumulation, which is necessary to initiate autophagy.
Based on this result, Dr. Yoshimori considered the idea that that autophagy is a phenomenon that removes damaged endosomes rather than identifying pathogens. If this hypothesis was correct, the reason why autophagy could eliminate various pathogens with different surface structures could be explained. When Dr. Yoshimori tested this hypothesis using artificial beads that could generate holes in endosomes, autophagy noticeably occurred, and the artificial beads were captured by autophagosomes. This made it possible to see how autophagy selects various decomposition targets.
A new path from unexpected results to discovery based on damage restoration
Next, he decided to elucidate the repair mechanism of lysosomes, an organelle similar to the endosome. He thought that, if his hypothesis was correct, restoration would proceed via the same mechanism as that of endosomes. Lysosomes play a role in the decomposition of intra-and extracellular components, and their interior is acidic and contains a variety of hydrolyzing enzymes. In autophagy, the molecule to be degraded is wrapped in an autophagosome, transferred to a lysosome, and decomposed.
When this lysosome is damaged, the acidic contents leak into the cytoplasm, causing inflammation, oxidative stress, and even cell death. For example, crystals of calcium oxalate accumulated in the kidney damage lysosomes in the cells of renal tubules and worsen the pathological condition of crystalline nephropathy. When he observed how the damaged lysosomes were actually repaired, he found that ubiquitination occurred, and autophagy selectively sequestered and repaired them as expected.
However, as the analysis proceeded, new findings were obtained. Lipidized LC3, which should be in autophagosomes, was also found in damaged lysosomes. A closer look at this unexpected result revealed that LC3 interacts with calcium channels that regulate calcium entry and exit on the lysosomal membrane, causing calcium to escape. As a result, TFEB, a transcription factor that regulates gene expression, is activated and repair progresses (Fig. 2).
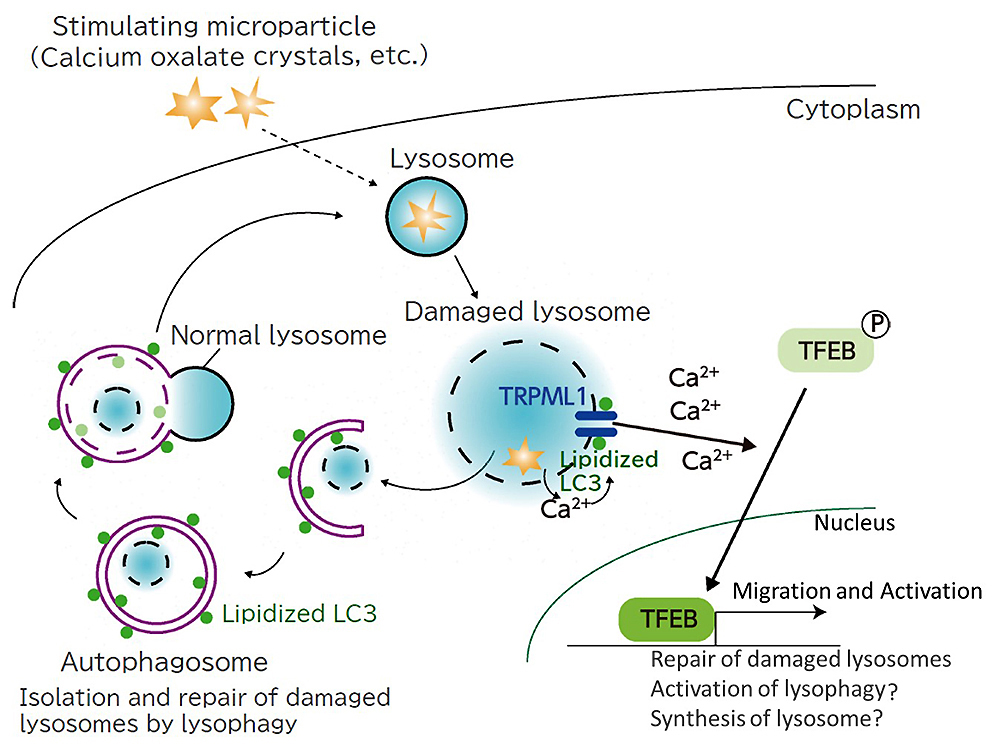
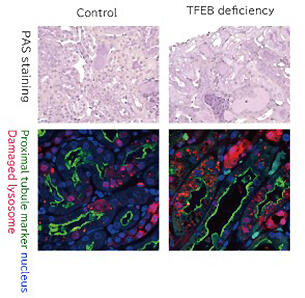
It became clear that LC3 works independently of the repair function of lysosomes in autophagy, and the results exceeded Dr. Yoshimori's expectations. "I would be happy if I could confirm that my hypothesis is correct, but it would be even more exciting if an unexpected phenomenon was confirmed. I think we got very interesting results this time as well," he said. When TFEB was disabled in a crystalline nephropathy mouse model, it was confirmed that the number of damaged lysosomes increased and nephropathy was exacerbated as compared with those in the normal crystalline nephropathy model mouse (Fig. 3). In the future, if compounds that act on the two pathways involved in lysosomal repair can be developed, this would be expected to lead to the treatment of various diseases associated with lysosomal damage, not limited to just crystalline nephropathy.
Establishment of a research center with a medical school to clarify the relationship with disease onset
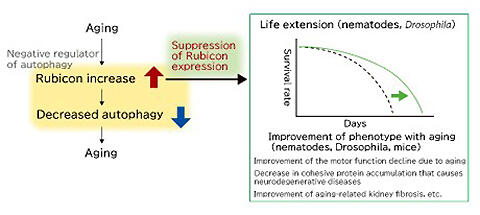
Dr. Yoshimori has had other great achievements. One is the discovery of Rubicon, a molecule that inhibits autophagy. Rubicon is a protein that inhibits fusion with lysosomes, the final stage of autophagy. To date, researchers have tried to promote autophagy, but if Rubicon, which acts as a brake, can be controlled, it might lead to drug development. "If we can reduce Rubicon levels, autophagy will occur more actively. We will find a way to leverage this to treat related diseases and extend healthy life expectancy," says Yoshimori, discussing the magnitude of the results.
In fact, recent studies have confirmed that Rubicon level increases with age, and autophagy is activated, and lifespan is extended in nematodes and Drosophila in which Rubicon has been inhibited (Fig. 4). Furthermore, in mice in which Rubicon is inhibited, the accumulation of fat in the liver and accompanying liver damage can be suppressed even if a high-fat diet is administered. Expectations and attention thus continue to increase (Fig. 5).
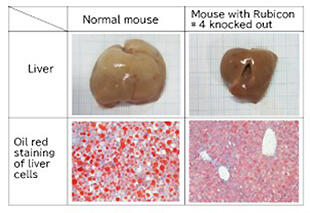
When the expression of Rubicon is suppressed, the size of the liver, which is enlarged with the high nutrition diet, returns to normal, and the accumulation of intracellular fat (red in the figure below) is suppressed.
The Autophagy Center attached to Osaka University School of Medicine, which was established in 2015, supports research that leads to the medical application of such research. Dr. Yoshimori is conducting joint research with the clinical classroom of the Faculty of Medicine, which specializes in various diseases. "Many medical teachers thought that this disease is related to autophagy. The establishment of the center has made it easier to conduct joint research and is producing new results," he says with a smile.
Not all life phenomena related to autophagy have been elucidated, but many people are waiting for associated disease remedies. Although the importance of basic research remains unchanged, verification of the results in clinical practice as soon as possible is required. "From now on, we will proceed with research in a two-pronged manner, without focusing exclusively on either basic or applied research," he says.
Building a research ecosystem - Reliable products for verification of effectiveness
Autophagy research is not limited to the cases mentioned above, and its application range has expanded greatly (Fig. 6). In response to these social expectations, Dr. Yoshimori established AutoPhagyGo, a venture company from Osaka University, in 2019 with the aim of implementing research results in society as quickly as possible (Fig. 7). As a technical advisor to the company, he supports research and development, and in July 2020, he launched the first product, "Autophagy Habit," a supplement jointly developed with UHA taste sugar to support healthy longevity. This is truly a rapid achievement of a real-world application.

To realize a healthy and long-lived society, joint research with multiple companies is still in progress, focusing not only on food products but also on cosmetics and dietary supplements. However, Dr. Yoshimori's aim is not limited to practical application. "At AutoPhagyGo, we are collaborating with multiple companies and aiming to establish a research ecosystem in which the profits obtained from practical application will be used for the next research fund," he says The company's funding is limited to only organizations and individuals that support this philosophy.
Until now, study at universities and other institutions has been conducted with research funding provided by JST and other public research support institutions. However, research funds are being reduced year by year, and many researchers are worried about new applications every day owing to the unstable employment system. "To continue to produce good results, it is important to sit down and study in a stable environment. To that end, I wanted to create a system that would allow me to solicit research funds from private companies and return the profits obtained from research into research funds; so I chose the form of a venture," he says, explaining the background behind this system.
To create this system, the Japan Autophagy Consortium was also established. Along with many academic researchers, including Dr. Yoshimori, 16 companies, including prominent companies from AutoPhagyGo and various industries, participate as corporate members. There, they share the results of cutting-edge research and disseminate valid information, based on science, to the general public, with the aim of obtaining credibility throughout the industry. "In order to gain trust, it is important to verify the effectiveness of the system," he says. "Throughout the industry, we will learn the latest research results from each other, and we will continue to develop technologies to measure the effectiveness of the system."
Thirty years have passed since Dr. Ohsumi's discovery in 1992. As a society, we are finally able to apply some of these results. However, this area is still a deeply interesting realm, where unknown areas remain. We will continue to watch the world of autophagy, which has fascinated many researchers such as Mr. Yoshimori, with a keen eye into the future.




