Bone, carbonate apatite, and hydroxyapatite
Bones consist of carbonate apatite [CO3Ap: Ca10-a(PO4)6-b(CO3)c], which contains 6-9 mass % carbonate. However, hydroxyapatite [HAp: Ca10(PO4)6(OH)2], which lacks a carbonate group, has been used in clinics as a typical artificial bone. The use of hydroxyapatite as an artificial bone was introduced in the 1970s. Since then, it has been widely used as a material that exhibits excellent osteoconductivity (the property of a material that bonds to bone when it is implanted in a bone defect). However, artificial bones made of hydroxyapatite exhibit inferior osteoconductivity compared to that of autograft. In addition, autograft is replaced by a new bone via bone remodeling (a biological process by which old bone is resorbed by osteoclasts and replaced with new bone formed by osteoblasts). However, artificial bones made of hydroxyapatite are not replaced by new bones. Therefore, the golden standard for reconstruction of bone defects is the autograft in which a bone is harvested from a healthy site of the patient oneself and transplanting it into the bone defect.
Artificial bone needs to be block or granular shape because powder induces inflammatory response regardless of its composition. Usually, sintering is employed for the fabrication. However, carbonate apatite containing a carbonate group undergoes thermal decomposition at the sintering temperature. In 1970s, hydroxyapatite which is similar to carbonate apatite but does not contain a carbonate group was found to be sintered. The sintered hydroxyapatite was found to exhibit osteoconductivity, and thus, has been used clinically as an artificial bone.
Bone formation in the living body occurs in the presence of water. In other words, bone is not formed by the sintering process but by chemical reaction. One of the chemical reactions we should learn from is "evolution". Invertebrates chose calcium carbonate [CaCO3] which can be taken from the sea water for their skeletal composition. During "evolution", vertebrates including humans are required to store phosphorus, which is necessary for energy metabolism, and chose to store it in their skeleton as carbonate apatite [CO3Ap: Ca10-a(PO4)6-b(CO3)c] which consist of not only calcium and carbonate but also phosphorous. I learned from "evolution", and found that a calcium carbonate block became a carbonate apatite block maintaining its macroscopic structure when immersed in an aqueous phosphate salt solution. This compositional transformation is caused by a dissolution-precipitation reaction.
Artificial bone composed of carbonate apatite
When osteoclasts were cultured on the surface of the carbonate apatite block, osteoclastic resorption pits were observed whereas pits were not observed on the surface of hydroxyapatite. Osteoclasts form Howship's lacunae and forms a weakly acidic condition. Under physiological conditions (pH 7.4), carbonate apatite is more stable than hydroxyapatite; however, under weakly acidic conditions, carbonate apatite becomes less stable than hydroxyapatite leading its dissolution. As results of presence and absence of osteoclastic resorption, hydroxyapatite is not replaced by new bone at all, whereas carbonate apatite is completely replaced with new bone over time. In addition, carbonate apatite artificial bone exhibits considerably high osteoconductivity compared to that of other artificial bones (Fig. 1).
Following non-clinical tests including histological evaluation, multi-center clinical trials were conducted for maxillary sinus floor augmentation to assess the function of carbonate apatite artificial bone. During the two-stage sinus floor augmentation surgery, biopsy (living tissue collection) can be performed because dental implants would be inserted at the bone which is replaced from the carbonate apatite artificial bone. Not only was 100% efficacy verified at all endpoints, such as bone formation and implant fixation, but replacement of the carbonate apatite artificial bone by a new bone was also confirmed in humans.
Based on the results of multi-center clinical trials, carbonate apatite artificial bone was approved for its clinical use in December 2017 as the world's first carbonate apatite artificial bone, and in February 2018, it was launched as "cytrans granules" by GC Co. Ltd. (Bunkyo-ku, Tokyo), which is the largest manufacturer of dental materials in Japan.
As mentioned above, in Japan, artificial bones were not allowed to be used for bone reconstruction related to dental implants or load bearing areas. Due to high osteoconductivity, carbonate apatite artificial bone gained approval for use in all dental fields including for bone reconstruction related to dental implants and for load bearing areas.
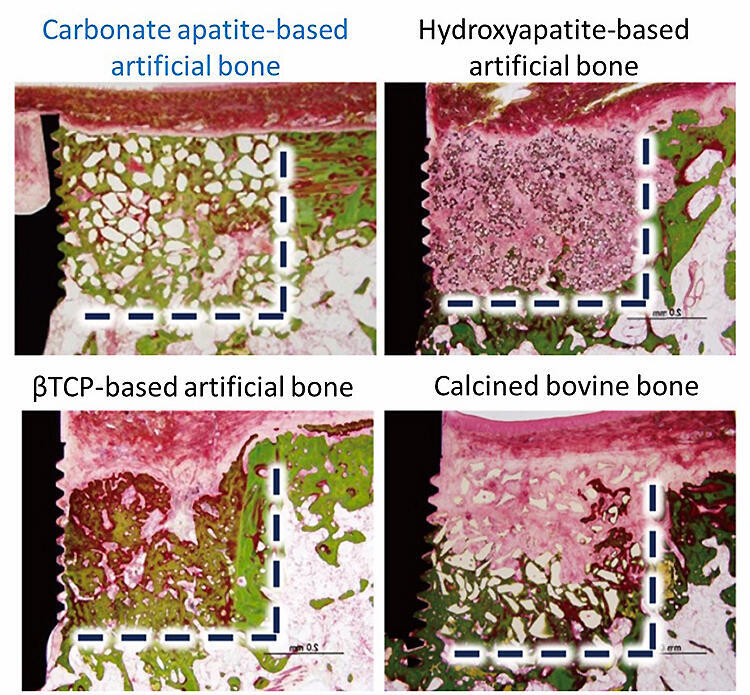
(Villanueva Goldner stain, green stained area indicates mature bone)
Artificial bone composed of carbonate apatite that surpasses autologous bone
The composition of bone was determined using autologous bone or, and based on this information, carbonate apatite-based artificial bone was developed. Carbonate apatite artificial bone exhibits osteoconductivity comparable to that of autograft, but it has not surpassed it yet. Compared to autograft, artificial bone has advantage with respect to its structure. Therefore, a carbonate apatite artificial bone with three- or one-dimensional pores was prepared. The three-dimensional pores were prepared by hydrate expansion reaction of calcium oxide spheres that were prepared by spray drying. Three-dimensional calcium hydroxide block, fabricated by the hydrate reaction of calcium oxide, was subjected to carbonation and phosphotization. Although its mechanical strength can be further improved, the three-dimensional artificial bone is replaced by new bone within 4 weeks of implantation owing to its three-dimensional structure. Artificial bones with one-dimensional pores (honeycomb structure) are prepared by extrusion molding. A binder containing calcium carbonate is extruded through a honeycomb die and further processed by debindering and phosphotization. Owing to the honeycomb structure, it shows extensive capillarity and can instantly extract blood at the site of the bone defect, among other functions.
Vertical bone augmentation, a technique for reconstructing the height of the jawbone that has decreased upon tooth extraction, is difficult to achieve even with autologous bone. However, it can be easily achieved using carbonate apatite-based artificial bone with the honeycomb structure (Fig. 2). The structure of carbonate apatite artificial bone can be easily controlled using a 3D printer. The structure as well as composition of artificial bone are important factors that determine bone regeneration ability. The goal of this research is to control structure of carbonate apatite artificial bone and obtain regulatory approval for its clinical use since it will surpass autograft with respect to functionality.
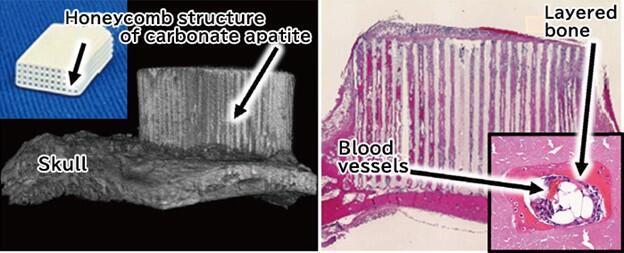
Left: Photograph showing carbonate apatite artificial bone with a honeycomb structure and micro CT image obtained 4 weeks after the procedure
Right: Histopathological image taken 4 weeks after the surgery (hematoxylin-eosin staining; red indicates mature bone; inset shows an enlarged image of a through-hole in a vertical cross section).

Professor Kunio Ishikawa
Department of Biomaterials, Faculty of Dental Science, Kyushu University




