There are great expectations for regenerative medicine and gene therapy as fundamental treatments for intractable diseases. However, there are several issues such as safety and sustainability of the effects that have prevented their full-scale practical use. TOKIWA-Bio (Tsukuba City, Ibaraki Prefecture) incorporates therapeutic genes into RNA using a completely different method than those used previously and has developed a technology for the stable expression of genes in the cytoplasm for a long period and is working on its practical application. By introducing genes safely and efficiently, the company is aiming to realize practical regenerative medicine and gene therapy and treat people worldwide who suffer from intractable diseases.

A virus identified in Japan becomes a breakthrough gene carrier
In the human body, proteins that perform various functions are produced based on genetic information. Therefore, genetic abnormalities can cause serious illness. The relationship between these genetic abnormalities and diseases has been rapidly clarified in recent years owing to the progress of DNA analysis technology developed in the 1980s. In addition, gene delivery technology that introduces ectopic genes into human cells and expresses them was also developed in the 1980s, and in the 1990s, clinical trials of gene therapy began as a medical application.
Gene therapy is a method that is used to radically cure a disease by administering a therapeutically effective gene to a patient, which supplements the function of the missing gene or adds a new function to the cell. Using a "gene carrier" called a "vector", such as an adenovirus or retrovirus, the administered gene is introduced into the cell. In other words, it uses the mechanism whereby genes related to the pathogenicity of the virus are replaced with therapeutic genes, and the virus infects cells.
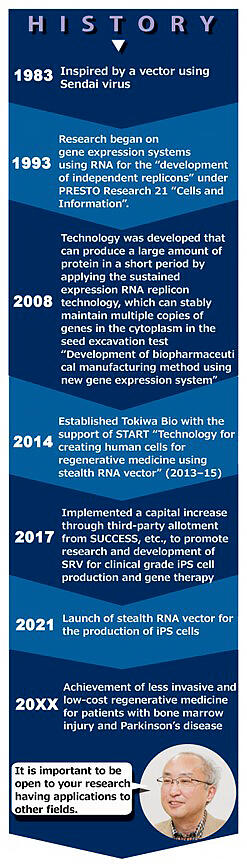
However, there are issues with existing viral vectors in that the persistence of gene expression is low, and this approach cannot be used if the gene to be introduced is too large or has issues with safety, such as the introduced gene entering the genomic DNA of cells and causing cancer. "I have been thinking about a system that enables stable gene expression without inserting genes into genomic DNA. One of them is 'Stealth RNA Vector (SRV)', which was successfully developed by taking inspiration from basic research on the Sendai virus", stated TOKIWA Bio's Director Mahito Nakanishi (Figure).
This Sendai virus is a historic virus that was first identified by the Japanese in the 1950s, and it is not pathogenic to humans and has the unique property of being able to fuse various animal cells with each other. SRV coexists without killing infected animal cells, and it is a new viral vector developed based on research on a mutant strain of Sendai virus (Cl.151 strain) that causes persistent infection. With SRV, by incorporating therapeutic genes into RNA rather than DNA, the introduced gene can be continuously expressed without affecting the genetic information of the cell. Furthermore, it is thought that it could be a truly revolutionary vector that carries multiple genes and large genes, which can be expressed simultaneously.
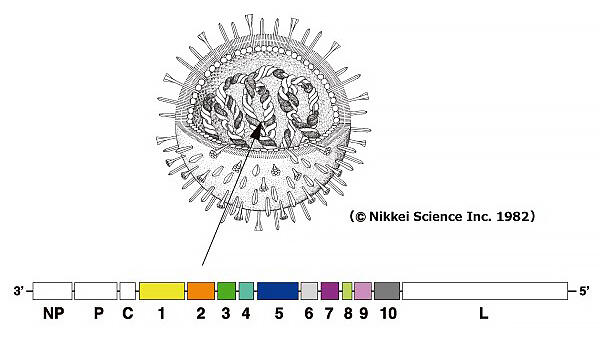
Adopts artificial RNA optimized for human cells to avoid interferon induction
Up to 10 loaded genes can be continuously expressed in the cytoplasm for a long period without introduction into chromosomes. Depending on the intended use, the optimal vector with the corresponding gene expression level can be selected. It is extremely safe because it is not carcinogenic, and it can be completely erased when it is no longer needed. It is one of the few purely domestic technologies in this field, and intellectual property is also registered in major jurisdictions around the world such as the U.S., E.U., and Japan.
A determination to "help many people" leads to clarification of the mechanism within 30 years of conception
Director Nakanishi, who has been studying the Sendai virus since he was a doctoral student, said that he began to envision gene-level therapies while engaging in basic research. At that time, even the methods for analyzing genetic information were under development, and gene therapy was a fantastical concept. Nevertheless, the reason why he never felt defeated was because of the words of a patient he met in 1983.
He said that it was a young woman suffering from familial hypercholesterolemia (FH) who said, "why can't you treat it even when you know the cause?". FH is a genetic disorder in which the receptors on cells that take up low-density lipoproteins in the blood are deficient, and this results in the early onset of myocardial infarction, but there is still no cure. He said, "I strongly wanted to put gene therapy with the Sendai virus into practical use as early as possible and help many people." This is how Director Nakanishi's quest began.
The breakthrough of this research was the successful decoding of the entire genomic RNA sequence of the Cl.151 strain and the clarification of the mechanism of persistent infection. This achievement, announced in 2007, paved the way for the creation of a Sendai virus vector suitable for gene therapy. By the time of the announcement of the predecessor of SRV, the "defective/sustained expression type Sendai virus vector (SeVdp vector)" in 2011, almost 30 years have passed since its conception.
Fateful encounter through the START program: Aiming for practical use with collaboration between the two individuals
"We know that the cause is a gene, and the number of diseases that can be diagnosed has increased. However, there are some intractable diseases for which there is no cure yet, and we have become more motivated to be involved in the development of cures", said President and CEO Masaharu Matsuzaki.
While working on the development of diagnostic methods for diseases at a private clinical testing company, Matsuzaki became interested in the development of treatment methods that result in a delay, rather than the spread, of a genetic disease. He has continued research on methods for safe introduction of a gene of interest into cells, the quality control of cells used for treatment, and transportation methods. However, there was a time when safe technology could not be found, such as deaths caused by gene therapy using viral vectors, which were the most powerful means.
Meanwhile, with inquiries from around the world, he met Director Nakanishi, who had applied for the Program for Creating Start-ups from Advanced Research and Technology (START) with the aim of putting SeVdp vector technology to practical use. "I was surprised that there was such a technology." Matsuzaki, who has experience in establishing and operating a research institute, immediately started collaborating with Director Nakanishi and started TOKIWA Bio in the second year of the adoption of START.
SRV, which was launched as a research reagent in 2021, is also effective for the efficient production of iPS cells, which require the transfer of four types of genes and sustained gene expression, known as Yamanaka factors, and this has already gained worldwide acclaim. In addition, the application to cell reprogramming technology, in which somatic cells are re-differentiated into cells of specific tissues and organs via gene transfer, is beginning to show signs of realization. They first plan to establish a foundation by spreading this in the research field, proceeding to the clinical trial phase toward the realization of gene therapy and regenerative medicine.
Matsuzaki is also looking at the next challenge. "In addition to the huge costs of clinical development, we also need experienced human resources and partner companies. It is also important to have a plan, such as which disease to first subject to clinical trials first." Nakanishi and Matsuzaki will continue to work together to improve the quality and stable supply of SRV.