Recently, researchers have dedicated considerable efforts to the depolymerization and reuse of plastics (chemical recycling) to solve the plastic waste issue, achieve sustainable development goals, and transition to a circular economy. To address the plastic waste issue, Professor Kotohiro Nomura of the Graduate School of Science at Tokyo Metropolitan University developed a chemical recycling protocol with low environmental impact using an original high-performance catalyst.

Use of calcium and titanium to address quality- and cost-related shortcomings
"Chemical recycling," which converts used plastics into raw materials, is a promising technology that can solve the plastic waste issue. However, chemical recycling is currently used for only approximately 3% of the total plastic waste worldwide. For example, chemical recycling of polyethylene terephthalate (PET) bottles requires large amounts of acids, bases, and additives and high temperatures. Therefore, chemical recycling presents several drawbacks, such as the difficulty in recovering the target material after reaction, wastewater treatment, and separation and purification of by-products from the polymer raw materials.
Nomura stated, "Current recycling has quality and cost shortcomings, and fabricating products from petroleum is less expensive than producing them via chemical recycling." As part of his contribution to the Japan Science and Technology Agency (JST)-CREST program on the "Development of Bio-Based Advanced Polymers and their Depolymerization, Chemical Recycle," Nomura developed high-performance calcium oxide and titanium complex catalysts that can degrade polyester and alcohols into raw materials via mixing and heating. Nomura and Professor Masafumi Hirano at the Institute of Engineering, Division of Applied Chemistry of the Tokyo University of Agriculture and Technology are also working on developing other catalysts.
The development of two catalysts prompted this research as part of the "Development of New Functional Polymers from Plant Oils by Efficient Catalytic Carbon-Carbon Bond Formation, Post-Modifications" study of the Strategic International Collaboration Research Program/e-ASIA JRP under the guidance of Nomura as the Principal Investigator. The study focused on the development of catalysts that promote the synthesis of high-value-added chemicals (i.e., fine chemicals), such as raw materials for polymers, detergents, and cosmetics, using inedible plant oil. Nomura commented, "The idea is quite simple: to use the typical catalysts for the ester exchange reaction, which converts plant oils into polymers, for the depolymerization of polymers through the same ester exchange reaction." The study results were published less than a year after the launch of the CREST program.
Another advantage is that the catalyst materials are calcium oxide and titanium complexes. Calcium oxide is a readily available and inexpensive commercially available catalyst with numerous industrial applications. Titanium complexes are also readily available and exhibit excellent catalytic performance. Therefore, upon heating polyester and alcohol mixtures, the alcohols can be removed, and 100% of the raw material can be recovered. Owing to its simplicity and high efficiency, the process can easily be developed into an environmentally friendly procedure with a low environmental impact.
Additionally, titanium complexes, which serve as ester exchange catalysts, remain intact after the depolymerization reaction. Therefore, polyester, which is degraded into raw materials, can be re-polymerized and re-converted into polyester, whereas the alcohols can be removed under vacuum. Accordingly, waste plastics can be converted into new plastics without changing the catalyst (Fig. 1 and Fig. 2). This method is expected to be used in numerous processes, such as for generating polymer raw materials from plant oils, synthesizing various fine chemicals, and converting plastic waste into high-value-added chemicals.
Fig. 1: Purpose and inspiration behind "chemical recycling" in this study.
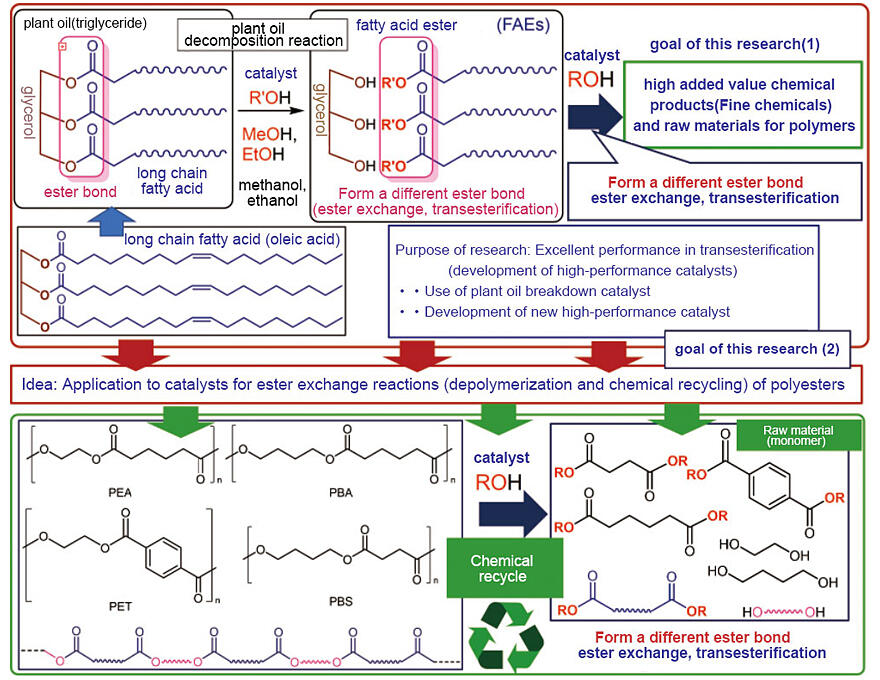
Fig. 2: Polymer depolymerization reaction.
Conducting original research based on industrial experience gained while working in the United States
Nomura began his research in the field of molecular catalytic chemistry during his Master's studies."After completing my Master's degree, I joined the Organic Synthesis Research Laboratory at Sumitomo Chemical Co., Ltd., where I was involved in the development of catalysts for the synthesis of fine chemicals. I reported my results and findings in my Ph.D. thesis. During my tenure, I was responsible for all the steps from catalyst development to the practical application of catalysts in the plants. My professional pathway is different from that of other professors in the field."
Thereafter, Nomura studied abroad at the Massachusetts Institute of Technology in the US, where he worked in the laboratory of Professor Richard Schrock, a Nobel Laureate in Chemistry, and performed research alongside many researchers who are currently based in the US and Europe. His 10 years of experience in the industry and the US and his collaborations with researchers in the US laid the foundation for Nomura's research activity. After returning to Japan, he joined a petrochemical research laboratory where he performed research on the practical applications of olefin polymerization catalysts, including ethylene, and on catalyst exploration.
Nomura joined the Nara Institute of Science and Technology in 1998. At the time, he had already decided on his future research direction. "To survive as a researcher, I thought it was crucial to design and synthesize highly original catalysts based on my own ideas and conduct research that could not be possible without those catalysts." Furthermore, he considered "pioneering an original precision synthesis technology and applying it to material development, taking advantage of its characteristics" as the technology adopted in this project.
From this background, he focused on "designing and creating original high-performance catalysts" and developed titanium and vanadium−niobium molecular catalysts. Although it was a time-consuming endeavor, Nomura identified numerous reactions that could not be performed without these catalysts. Nomura's dedication and numerous research achievements have been recognized. He received the Technological Progress Award from the Chemical Society of Japan in 1996 and the Society Award for Technical Achievement from the Catalysis Society of Japan in 2001. He also received the Society Award for Academic Achievement from the Catalysis Society of Japan in 2019.

Joint project with researchers in Thailand and the Philippines: Undertaking the challenge of using inedible plant oils
Nomura assumed a professor position at Tokyo Metropolitan University in 2010. His research team, which has strong connections with Chulalongkorn, Mahidol, and Thammasat Universities in Thailand, has included numerous international students and has published numerous papers. Moreover, his research team jointly planned and hosted an international conference in Bangkok. In view of this background, while exploring collaborative research opportunities, the team in Thailand invited researchers at Ateneo de Manila University in the Philippines to join Nomura's team. The international team focused on the theme of synthesizing new functional polymers from inedible plant oils through catalytic reactions, and the project was approved by the e-ASIA JRP.
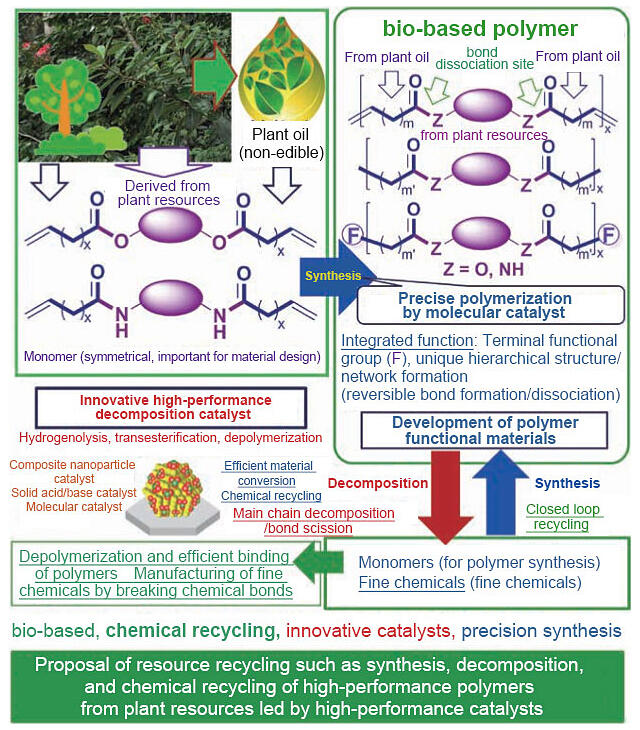
As part of the e-ASIA JRP, researchers from Thailand and Japan collaborated to develop a catalyst for the efficient synthesis of chemicals from inedible plant oils. The researchers in Japan were responsible for synthesizing bio-based polymers, whereas those in Thailand and the Philippines were responsible for evaluating the functionalities of the biopolymers. Subsequently, catalyst development for ester exchange reactions, one of the outcomes of the e-ASIA JRP, was added to a CREST project, leading to a breakthrough in plastic depolymerization.
The primary objectives of CREST are the development of high-performance polyesters derived from plant resources and chemical recycling. "Because depolymerization is an ester exchange reaction, which we studied during the e-ASIA JRP, we initially worked on developing a high-performance catalyst that can degrade commercially available polyesters while focusing on addressing the current shortcomings," Nomura stated (Fig. 3). Researchers worldwide have dedicated extensive efforts to degrading inedible plant oils and plant components that do not compete with edible crops into new functional polymers that can be recycled."The number of researchers in Japan who focus on developing polymers is considerably smaller than that of the researchers focused on other compounds or mixtures, such as jet fuel," said Nomura, explaining the essence of his research.
Fig. 3: Outline of the CREST project.
Proposal for the development of materials with diverse functionalities
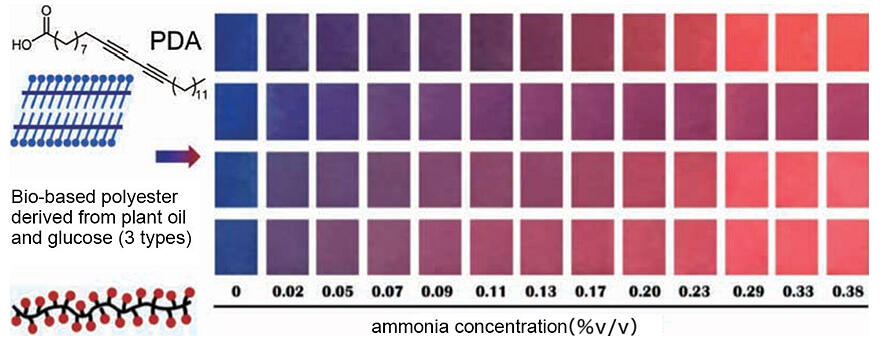
In addition to developing precision synthesis methods, Nomura is currently engaged in research on functionality development. The impetus for this research was the International Joint Research Program of the e-ASIA JRP. Upon evaluating its functionality in a research laboratory in Thailand, it was determined that the synthesized polymer functioned as a sensor capable of detecting several substances, such as ammonia and ethylene (Fig. 4). This discovery prompted further research collaborations with researchers in Thailand on the potential use of the polymer as a food packaging film, which can serve as a freshness sensor.
Fig. 4: Polymer composite material with sensing functionality (the composite material comprises polydiacetylene (PDA) and a bio-based polyester).
The researchers in the Philippines focus on developing water purification membranes because the synthesized glycopolymer and chitosan composite films can effectively trap contaminants, such as mercury, in water. "Such ideas that can lead to practical applications could not have been inspired by the Japanese researchers alone. These achievements are unique to an international joint research team," said Nomura, emphasizing the significance of international joint research.
However, joint research projects with ASEAN countries have not always been effortless, owing to the enforcement of citywide lockdowns in various areas because of the COVID-19 pandemic in 2019.
"We could not send samples overseas because the university was closed; therefore, we had to send samples to the homes of our international collaborators." Nomura recalls, "We had hoped to invite international students to Japan to conduct research and interact with each other. However, that was postponed."
At the same time, the team took advantage of the opportunities and communication approaches unique to the COVID-19 pandemic. "We had already established a research policy and shared it with the research teams; therefore, we were able to communicate well via web meetings. Previously, we faced numerous constraints imposed by distance and time limitations. However, we were able to attend numerous academic conferences and international meetings online during the COVID-19 pandemic." Furthermore, owing to an increase in time availability, our team members were able to delve into a wide range of topics related to their field of research.
Lengthening polymer chains to overcome practical application barriers
Nomura considered the subsequent polymerization of the as-synthesized polymer as a challenge in advancing the project. "Our research focuses on synthesis methods and catalysts. The polymer chains must be lengthened to use the as-synthesized polymers in practical applications." To address this issue, he focused on the olefin metathesis polymerization of various monomers derived from plant oils and glucose.
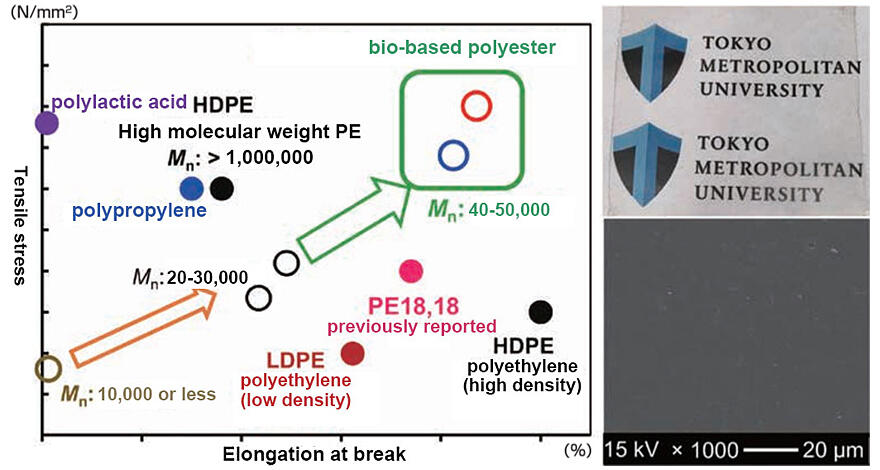
Materials with tensile strength and elongation at break superior to those of general-purpose polyethylene were fabricated owing to the efforts dedicated to developing a method for synthesizing high-molecular-weight polymers (Fig. 5). This achievement resulted from collaborative research with Dr. Hiroshi Hirano at the Osaka Research Institute of Industrial Science and Technology, a member of Nomura's research team. This has become a critical technology for advancing the research on bio-based high-performance materials that can be degraded and recycled.
Fig. 5: Mechanical properties of bio-based polyesters.
Furthermore, the research team has focused on developing vanadium and niobium complex catalysts. Nomura commented on the future prospects: "Although it was demonstrated that the catalytic activity, heat resistance, and selectivity control of vanadium and niobium complex catalysts are superior to those of other catalysts, further improvements are still necessary for their application for this task. In the future, I hope that the studies on the catalysts and materials that we have been developing will converge toward a unified outcome."
Many researchers in the field of catalyst development are based in foreign countries, such as European countries and the US. Nomura would like to continue to actively promote international joint research projects. Furthermore, although several foreign researchers have developed catalysts with practical applications, overcoming the practical application barrier is a challenge for researchers in Japan. Nomura is focused on overcoming these barriers by expanding his knowledge in a wide range of fields in addition to his accumulated experience.

(Text: Sachiko Ito, Photo: Hideki Ishihara)




