"Genome medicine"—the medical approach where all genetic information which differs from individual to individual is utilized for the diagnosis and treatment of diseases—is being widely practiced in Japan. There is great hope for this new medical care technology, as it has the potential to identify effective treatments that are tailored to each individual patient. Simultaneously, it has been pointed out that as genetic information is the "ultimate privacy" and does not change throughout life, its protection and prevention of unjust discrimination is a prerequisite for the advancement of this medical care approach.
The Genome Medicine Promotion Act—a law to promote this approach in an appropriate, fair, and just manner—was enacted in the 211th ordinary Diet session on June 9. The Act sets forth the ideals to achieve the world's highest level of genome medicine so that the public can widely enjoy its benefits. It also mandates the government to formulate a basic plan and specifies that there should be no unjust discrimination based on genetic information. Effective measures are expected to be taken to establish a research and medical care system that realizes the ideals of the new law.
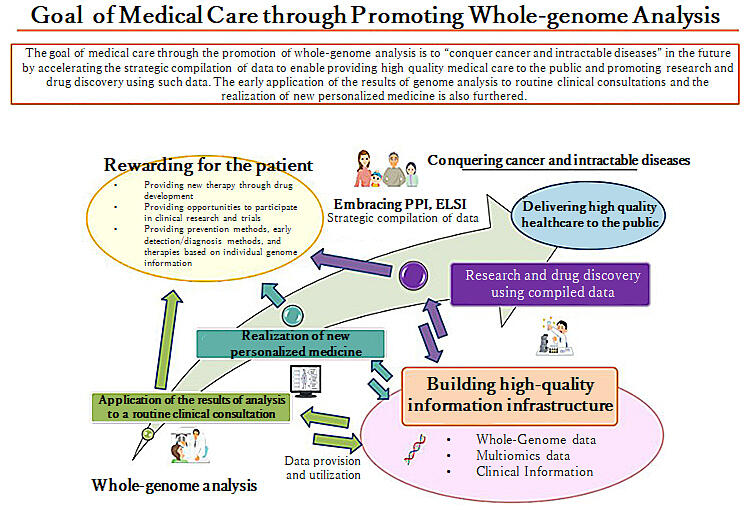
(provided by the Ministry of Health, Labor and Welfare and translated by Science Japan)
A Blueprint for Building a Body
Genome is an English word which indicates all the genetic information contained in an organism. The word genome is coined by combining the word gene and the suffix -ome, which means "a whole." Some say that it is derived from the ending of the word "chromosome." This includes information that specifies the types of proteins for making up the body of an organism as well as those that control the functioning of other genes.
In most living organisms, with the exception of some viruses, genetic information is encoded in a sequence of four types of chemical building blocks called "bases" that make up DNA. It may seem confusing when the terms genome, gene, and DNA are juxtaposed, but genomes are defined as "all genetic information which contains genetic information expressed by the sequence of DNA bases."
In the 1990s, the U.S. and other industrialized countries launched genome sequencing projects to decode the entire human genome. In April 2003, the leaders of the U.S., the U.K., Japan, France, Germany, and China declared the completion of the Human Genome Project. An international team comprising researchers from six countries announced that they decoded the sequence of about 2.83 billion nucleotides in the human genome, which consists of approximately 3.2 billion base pairs.
Twenty-four institutions from six countries participated in the decoding, including RIKEN, Keio University, and Tokai University in Japan, which contributed up to 6% of the total decoding work. What surprised the world then, in relation to the results of decoding, was that the only 2.6% of the DNA sequence within the more than 3 billion base pairs was genetically relevant. The remainder were segments that did not provide a cell with any instructions to make a specific protein.
The body is composed of cells. For example, muscles are made of muscle cells, and bones of bone cells. Every single cell contains genes, and the genome is like a blueprint, so to speak, for building the body. The products of decoding genomes have had a great impact on the subsequent progress of medicine and medical sciences.
Technologies in genomics have also made great progress. Dr. Emmanuelle Charpentier of the Max Planck Institute in Germany and Dr. Jennifer Doudna of the University of California, Berkeley, U.S., who developed "CRISPR-Cas9," a tool to efficiently edit the genome of organisms, and published a paper on this development in 2012, won the Nobel Prize in Chemistry 2020.
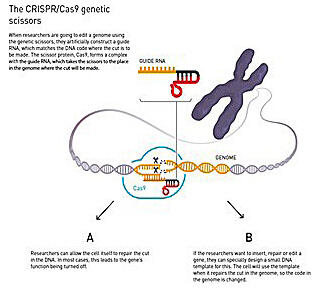
(provided by the Nobel Foundation/Royal Swedish Academy of Sciences)
Earliest practical application in the field of cancer treatment
A change in a gene constitutes a genetic mutation, and which gene mutates varies from individual to individual. Genome medicine comprehensively studies genetic mutations, and by taking advantage of the results, diagnoses and treats disease effectively and efficiently, in a manner suited to individual patients.
Since the 2000s, following the groundbreaking achievement of decoding the human genome, it has become clear that genetic mutations are involved in the onset and development of a variety of diseases. Research on the development of technologies to screen for genes and drugs to treat genetic abnormalities has also progressed.
Genome medicine was expected to be applied to the treatment of a variety of diseases, including intractable diseases, but it was first applied in practical medicine in the field of cancer treatment. The domestic medical infrastructure in Japan has rapidly developed over the past five years under an initiative of the Ministry of Health, Labour and Welfare (MHLW) and the National Cancer Center (NCC). A medical care system referred to as "Cancer Genome Medicine" is now in place.
Cancer is a disease in which genes in normal cells are altered to become cancer cells, and the proliferating cancerous tissue debilitates the body. According to NCC, cancer genome medicine targets cancer tissues by simultaneously analyzing a large number of genes to spot genetic mutations and aims to tailor treatments to each individual's constitution and medical condition.
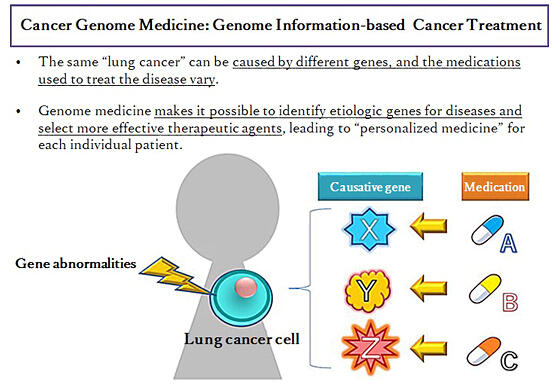
(provided by MHLW)
Gene panel testing now covered by insurance
In cancer genome medicine, which was put to practical use early on, testing for genetic changes occurring in the tissues of cancer patients plays an important role. As of June 2019, the "Cancer Gene Panel Test," analyzing many genes at once and basing treatment on its results, is covered by insurance.
The panel test is available at a total of approximately 250 medical institutions (as of April 2023), including 13 Core Hospitals for Cancer Genome Medicine and 32 base hospitals designated by MHLW, as well as over 200 Cooperative Hospitals. If the panel test results reveal genetic mutations and there are medications that are expected to be effective against the mutations, viable medication treatments, including clinical trials, are considered. In addition to primary physicians and genetic medicine specialists, health care professionals with counseling skills also participate in drawing up a treatment plan.
However, according to NCC, there are some cases in which the test results do not reveal any genetic mutations, or in which there is no available medication even if a mutation is spotted. Only about 10% of patients actually reach the stage of taking medications. Improving this figure is one of the challenges for the future.
Expecting the potential to mitigate side-effects
Currently, numerous anticancer drugs are administered in the standard therapy for cancer. Anticancer drugs also damage normal cells, and many patients suffer from serious side effects of these drugs. If different patients have different mutated genes at the same cancer site, the possibility arises that the same medication may work differently or have different side effects. If the genetic mutation is identified, that part of the body can be targeted with the specific medications, thereby reducing side effects and achieving a strong therapeutic effect. "Molecular targeted therapies," which attack proteins and other molecules specific to an individual patient's cancer, are already being used in clinical practice.
The practical application of genome medicine in the field of cancer treatment is accelerating, but more patient data needs to be accumulated to improve treatment performance. In parallel, data related to test results and medical conditions should be under strict controls. This situation has led to the protection of information and prevention of genetic discrimination emerging as major issues in Japan, resulting in the recent passage of the Genome Medicine Promotion Act.
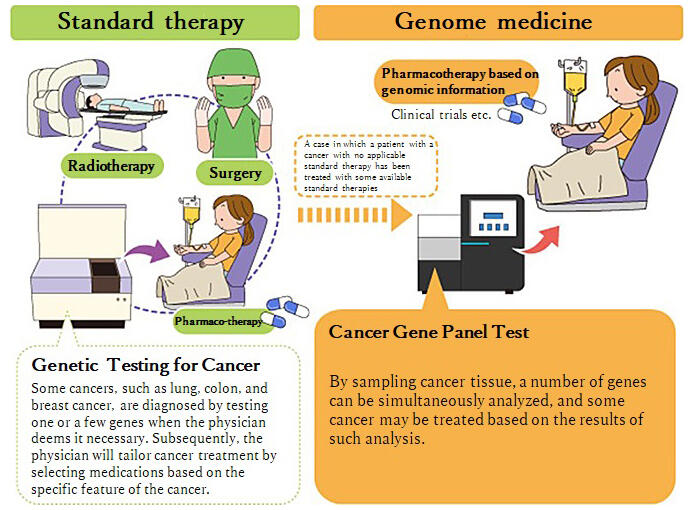
(provided by NCC)
Bipartisan bill submitted and passed with haste
Genetic information, which is useful in diagnosing diseases and prescribing treatments, may turn out to be the cause of an eventual genetic discrimination that, depending on how it is dealt with, may lead some people to be disadvantaged in employment or eligibility decisions regarding insurance at the time of searching for a job or enrolling in health insurance, respectively. Unless patients can provide their genetic information without any concerns, the sound development of genome medicine will not be assured in the future. In April 2022, the Japanese Association of Medical Sciences (JAMS) and the Japan Medical Association (JMA) issued a joint statement calling on the government to legislate to prevent social disadvantage and discrimination on the basis of genetic information. Patient associations and other groups made similar statements.
In response to these movements, the "Diet Members Caucus for the Improvement of the Social Environment for the Promotion of Appropriate Genetic Medicine" (chaired by House of Councilors Member Hidehisa Otsuji), a bipartisan group of Members of Parliament from the ruling and opposition parties, brought forward an outline of a legislative proposal in October 2022 that called for establishing a system and financial support from the government, as well as forming a legal framework to prevent discrimination. The bill was introduced in the plenary session of the House of Representatives on May 31, 2023.
Although the bill was passed by the plenary session of the House of Councilors on June 9 and enacted quickly, Japan's legislation and regulations regarding the medical application of genome information still lag far behind those of Europe and the U.S. The principle that the dignity of the individual should not be compromised regardless of their genetic characteristics was internationally shared in the "Universal Declaration on the Human Genome and Human Rights" adopted by the United Nations Educational, Scientific and Cultural Organization (UNESCO) in 1997. In the year 2000, the Japanese government also established "Basic Principles on Human Genome Research" to promote related research without undermining human rights and dignity; however, the actions for the necessary legislation or regulation in this regard were not initiated.

(provided by the Secretariat of the House of Representatives)
In the U.S., the Genetic Information Nondiscrimination Act (GINA) was passed in May 2008, prohibiting discrimination in employment and insurance enrollment based on genetic information. A bill with a purpose similar to that of the above-stated law had been introduced to the U.S. Congress as early as 1995, but it was repealed owing to opposition from industry. It was subsequently submitted and repealed several times before it was passed. Canada has a law similar to GINA. In addition to France and Germany, South Korea also prohibits discrimination through laws regarding genetic testing, among others.
Healthcare Promotion and prevention of discrimination are an inseparable pair
The Genome Medicine Promotion Act, which was finally enacted in Japan, has three basic principles: public benefit, bioethical integrity, and protection of information and prevention of discrimination. Among the most important provisions, the anti-discrimination clause clearly stipulates, "Necessary measures shall be taken to engage in prohibiting unjust discrimination based on individual genome information that is inherent at birth and can be passed on to offspring, as well as coping with other matters that may arise from the wider use of the said genome information."
Genetic mutations that increase the risk of diseases may occur in everyone. Genome medicine has the potential to benefit everyone in the form of advances in diagnostic and treatment methods. However, simultaneously, discrimination based on genetic information is not someone else's problem. Forced genetic testing is unacceptable, and the freedom not to undergo a genetic test should be guaranteed.
Technological progress in the medical field is accelerating. Society has high expectations for the development of new diagnostics and therapeutics, but genome medicine is not viable for this purpose unless data are obtained from a large number of people. The promotion of medical care and the establishment of mechanisms of protecting genetic information and preventing discrimination and disadvantage are two sides of the same coin.
(provided by the Secretariat of the House of Representatives)
(UCHIJO Yoshitaka / Science Journalist, Kyodo News Visiting Editorial Writer)
Original article was provided by the Science Portal and has been translated by Science Japan.




